For children with type 1 diabetes, our group developed and validated a 9-item interview to assess risk for poor glycemic control in newly-diagnosed type 1 diabetes patients, the Risk Index for Poor Glycemic Control (RI-PGC; Schwartz et al. 2013a). The measure was shown to have good sensitivity and specificity for poor glycemic control (A1c 9.5 %), and was also able to identify patients at risk for DKA . Most importantly, it was designed for easy use and scoring by physicians and other medical providers. The nine items are scored as a simple sum which translates directly into an estimation of the absolute increase in risk associated with that score (Table 12.1).
Table 12.1.
Use of the Risk Index for Poor Glycemic Control (RI-PGC) to estimate absolute increase in risk for poor glycemic control (HbA1c 9.5 %), emergency room (ER) visits, and diabetic ketoacidosis (DKA). Predicted values are approximations accurate to within 10 %. All values are rounded to the nearest multiple of five for ease of use. (From Schwartz et al. 2013a)
RI-PGC score | Poor glycemic control (%) | ER visits (%) | DKA (%) |
|---|---|---|---|
0 | + 0 | + 0 | + 0 |
1 | + 10 | + 5 | + 5 |
2 | + 20 | + 15 | + 20 |
3 | + 35 | + 25 | + 20 |
4 | + 40 | + 30 | + 30 |
5 | > + 40 | > + 30 | + 40 |
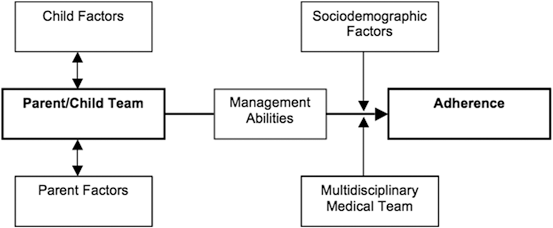
A more extensive interview that provides broader coverage of psychosocial risk factors has also been developed. The Psychosocial Risk Screening Measure (PRiSM) is a 36-item semi-structured interview assessing risk in five domains known to be important risk factors for poor diabetes control (Schwartz et al. 2010): sociodemographic factors such as race/ethnicity and SES; child problems (e.g., behavior or mood problems); family conflict; caregiver problems (e.g., depression); and anticipated diabetes-related problems (e.g., anticipated conflict over diabetes management). These different domains have been organized into a “simple model” of risk for nonadherence (Fig. 12.2) that was used to guide development of the PRiSM screening tools (for a detailed description of the initial development of the screening tool, see Schwartz, Axelrad et al. 2011). The model assumes that the critical “actor” is the child/parent team, whose management abilities directly affect adherence, although they are also influenced by environmental factors and the healthcare team.
The PRiSM has been field-tested for feasibility and acceptability (Schwartz, Cline et al. 2011) and is currently being validated. A comprehensive training manual (and a supervisor’s guide) that provides detailed instructions for using the RI-PGC and the PRiSM has been peer-reviewed (Schwartz et al. 2014) and is available for free download from MedEd Portal at www.mededportal.org/publication/9643. Moreover, we are also now developing modules for use with other pediatric populations, beginning with children with cancer.
Can problems be reliably identified after diagnosis?
In established patients, there are many evidence-based assessment tools for nonadherence that have been validated in pediatric patients (see Quittner et al. 2008 for review). Typically these are questionnaires that are completed by the patient (if old enough) and/or the parent.
Psychosocial screening tools can also be used to detect risk for nonadherence when there is a well documented relationship between the risk factor and the outcome. For example, Hilliard et al. (2011) used brief, validated measures of depression (Children’s Depression Inventory) and anxiety (the state scale of the State-Trait Anxiety Inventory for Children) to predict adherence (blood glucose monitoring frequency) and glycemic control (HbA1c) in youth age 13–18 with type 1 diabetes 1 year later. Symptoms of depression predicted reduced blood glucose monitoring, and anxiety predicted poorer glycemic control, possibly due to concurrent associations with stress.
Healthcare providers can also assess for nonadherence more informally. While informal assessment is more prone to individual biases and lack of sensitivity to uncovering problems, it is often the only option available to clinicians in busy routine practice. Moreover, simply asking patients about current problems can be valuable in its own right, as it has been shown to relate to an improved therapeutic relationship. Healthcare providers can start by acknowledging that most patients struggle with managing aspects of the medical regimen, and then ask what parts of the regimen are difficult for the patient. Assessing specific behaviors (e.g., frequency of blood glucose checks) is critical. Providers can also ask more general questions about the burden of illness management. Peyrot and Rubin (2007) suggest asking patients:
Do you feel overwhelmed or burned out by the demands of illness management?
Do you get the support you need from your family for illness management?
A downside of informal questioning is that it can be difficult to interpret findings and compare findings across patients, and important areas might be missed.
Is screening feasible and acceptable to patients and their families?
Feasibility of screening is threatened by its potential burden on patients/families, healthcare providers, and the system of routine care. As noted earlier, screening services are often not reimbursable, and they can be quite resource-intensive in terms of administration, scoring, and interpretation. To address these problems in screening newly diagnosed cancer patients, Kazak et al. (2011) arranged for nursing staff to administer the measure to patients, who completed it as a questionnaire. They reported an 88 % completion and return rate, and 98 % of cases were scored, reviewed, and shared with the treatment team within a couple of days. They also reported a high degree of buy-in from nursing leadership and staff.
We took a different approach to screening newly diagnosed type 1 diabetes patients. For clinical reasons, we believed it was important to use a face-to-face interview approach rather than a questionnaire. Conducting interviews allowed us to provide appropriate support to families throughout the assessment process; a secondary goal was to put a “face” on psychology to reduce potential stigma (Schwartz, Axelrad et al. 2011). To staff this service, we incorporated the screening into our training program for pediatric psychology pre-doctoral interns and fellows. In an initial feasibility study (Schwartz, Cline et al. 2011), we were able to screen 75 % of patients, with an almost 97 % participation rate (121 out of 125 families approached). A subset of families (n = 30) completed satisfaction ratings, with a satisfaction score of 90 %, reflecting an average rating of 4.5 out of 5 (Very Good to Excellent). No one rated the service “fair” or “poor.”
Is there effective intervention for identified problems?
As discussed in Chap. 4, there are a multitude of effective, evidence-based approaches to treating nonadherence in pediatric patients that also have beneficial effects on children’s health. They do tend to be resource intensive, however, typically requiring implementation by a well-trained behavioral health specialist (although there are exceptions; Anderson et al. 1999). As a result, most of the proven interventions have been focused on patients with clinically significant needs. Interventions at different levels of risk are clearly needed.
There are also effective interventions for related psychosocial concerns such as depression. For example, in a meta-analysis of intervention studies targeting internalizing problems (e.g., depression, anxiety), externalizing behavior problems, coping skills , and health beliefs in children with cancer, cystic fibrosis, sickle cell disease, and type 1 diabetes, Beale (2006) found a mean effect size of d = 0.71 across illness types, which he noted translates into improvement for 80 % of participants.
Concerns About Screening
As noted by many authors, universal screening raises a number of important ethical issues. First is the issue of informed consent. We believe it is very important for families to understand why a screening is being conducted, and to provide informed consent (and assent for minor children) for the procedure. To this end we have developed very stringent consent procedures around our diabetes screening process, as detailed in the training manual (Schwartz et al. 2013).
Second, many providers express concern about asking about psychosocial risk factors at or near the time of disease diagnosis, which is usually a fraught and stressful time for most patients and their families. This is an understandable concern, though we believe it is probably unfounded in most cases. In our experience screening newly diagnosed children with type 1 diabetes (over 600 patients to date), very few families have had any sort of complaint; instead, the vast majority have commented on how helpful it was to have someone to talk to about these concerns. It did help that our interviewers were all well trained in psychological interviewing and in helping families manage stress.
Third is the issue of false positive and false negatives. False positives are often a concern in medicine, as a false positive test can result in unnecessary procedures, treatments, and expenses, potentially placing a substantial extra burden on patients and their families. In addition, false positives can be stressful and frightening, as when a person is told he has a condition with significant long-term implications. These are important considerations. However, we would argue that the problem of false positives is small if not negligible in the realm of adherence. The usual outcome of a positive result is a conversation between the clinician and the patient and parent, in which it can be determined whether the family is interested (and sees a need) for further evaluation and/or treatment—hardly a bad thing.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree