Robotic technology as applied to laparoscopy utilizes robotic technology to facilitate the performance of the operation by placing a computerized interface between patient and surgeon. Although originally designed for cardiovascular surgery and approved for that use by the U.S. Food and Drug Administration (FDA) in 2003, it has found applications in urology and gynecology, and more recently in otolaryngology and colorectal surgery.
There are studies showing that the use of robotic technology for the performance of preset laboratory exercises results in faster performance times, increased accuracy, enhanced dexterity, easier and faster suturing, and a lower number of errors when compared to conventional laparoscopic instrumentation (1–4). The physical stress for medical students learning robotics is significantly less than what they encounter when learning the same tasks by laparoscopy (5). The technological advantages of robotics can facilitate learning surgical techniques when compared to acquiring similar skills in laparoscopy, as demonstrated by a shorter learning curve for robotic surgery. There is a flattening of the operating time after 20 to 50 robotic hysterectomies, while for laparoscopically assisted vaginal hysterectomy (LAVH) a similar facility with the requisite skills does not occur until after 80 procedures (6–9). In gynecologic oncology, a flattening of the operating time was noted after 20 procedures for endometrial cancer when using the robotic technology (10).
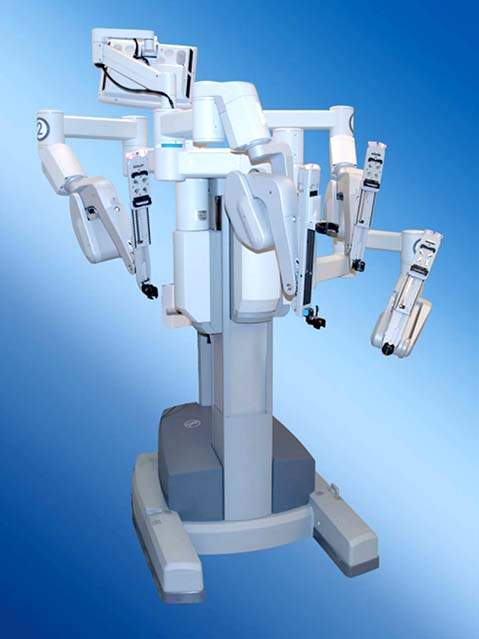
Figure 25.1 Robotic column with robotic arms.
Robotic Technology and Differences with Laparoscopy
There is only one robotic system available, brand-named Da Vinci (Intuitive Inc, Sunnyvale, CA), which received FDA approval for the performance of hysterectomy in 2005. A second generation of the device, the Da Vinci S, was released in 2006, and the third generation of the Da Vinci robotic system, the SI model, was released in 2009 and incorporates a resident teaching console. A robotic system is composed primarily of a robotic column, which holds the robotic arms (Fig. 25.1) and a surgeon’s console (Fig. 25.2). The robotic arms that hold the robotic instruments (Fig. 25.3) are fastened to the robotic trocars (Fig. 25.4). Commonly used robotic instruments for gynecologic surgeries are shown in Figure 25.5A-F.
Figure 25.2 Robotic console with hand controls and foot switches.

Robotic Column
The robotic column has three or four robotic arms, depending on the selection at the time of purchase (Fig. 25.1). The fourth arm is extremely useful for assisting with tissue retraction. The robotic arms always function in the direction toward the robotic column and not away from it. For this reason the robotic column must be positioned strategically in response to the location of the surgery that will be performed.
For pelvic surgery the robotic column is commonly situated between the patient’s legs (Fig. 25.6), while for upper abdominal surgery it is positioned at the patient’s head. For procedures to the right or left of the abdomen, the robotic column is positioned at the opposite side of the patient. For instance, for extraperitoneal left aortic lymphadenectomy, the robotic column is at the patient’s right side. For procedures where easy access to the entrance of the vagina and rectum is necessary, the robotic column is positioned lateral to the right or to the left of the patient’s legs.
Figure 25.3 Robotic laparoscope and two robotic arms with robotic instruments inserted. The tip of the instruments can be appreciated in the insert.
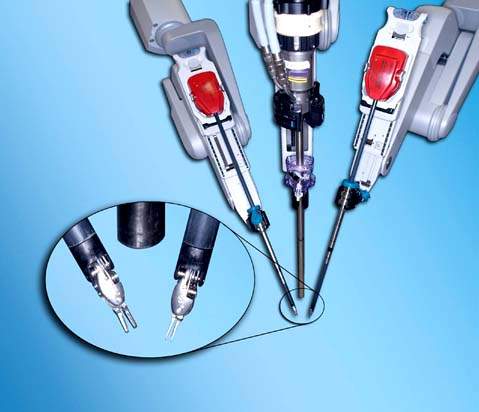
Figure 25.4 The robotic trocars are metallic and can be seen to the right of the picture. The escape valve was added because the original trocars did not have it. The three types of available trocar obturators are on the left of the picture: blunt (left), tissue-spreading (middle) and cutting (right).

Figure 25.5 Robotic instruments commonly used in gynecologic surgeries. A: Monopolar curved scissors. B: Monopolar spatula. C: PK (plasma kinetic) dissecting forceps, bipolar. D: Prograsp forceps, bipolar. E: Tenaculum forceps used for myomectomy. F: Needle grasper used for suturing.
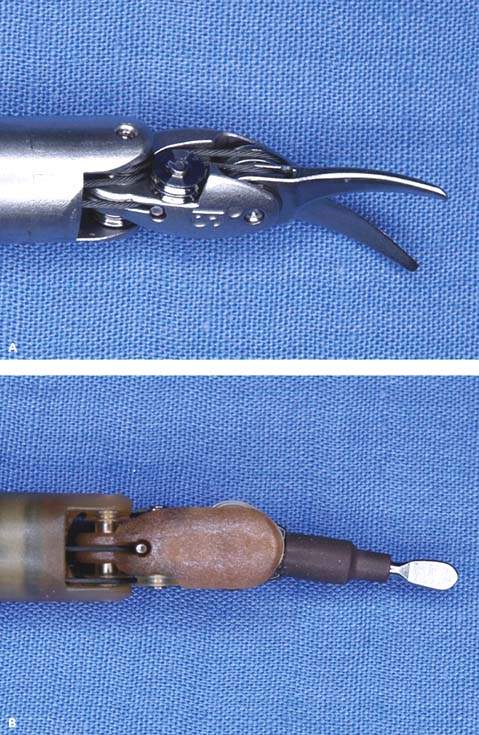
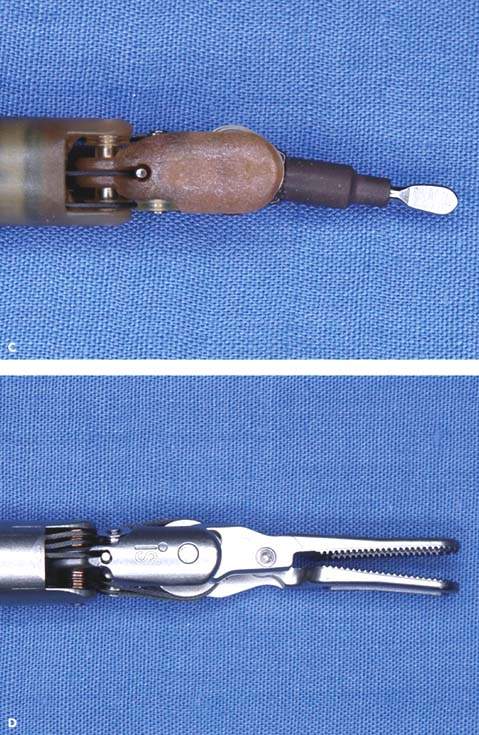
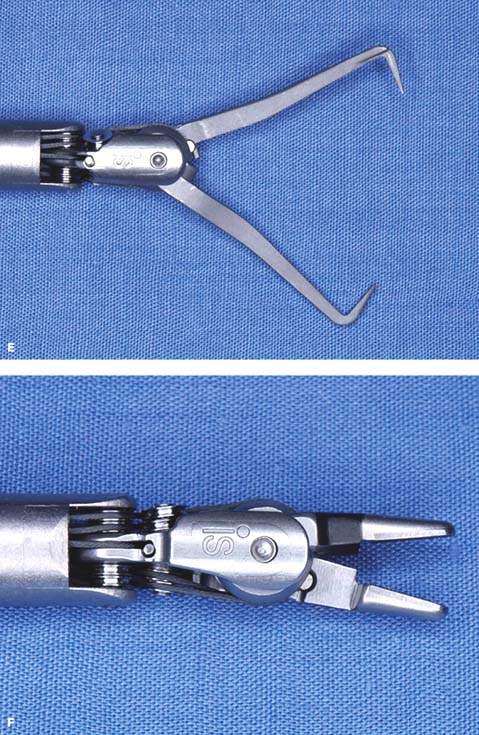
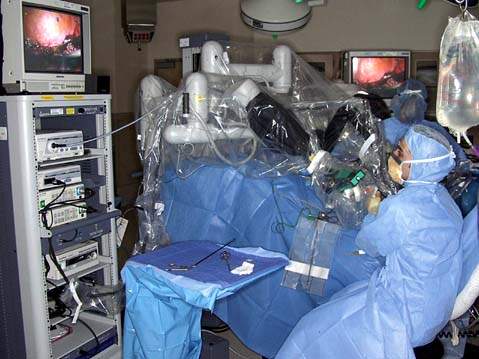
Figure 25.6 The robotic column is usually positioned between the patient legs for pelvic surgery. The assistant is seated to the left of the patient and is scrubbed for the procedure.
Robotic Console
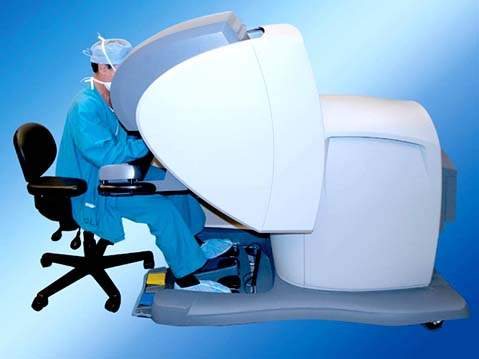
The surgeon sits at the console (Fig. 25.2) away from the patient (Fig. 25.7) and the assistant sits next to the patient (Fig. 25.6). The surgeon has a stereoscopic image, which is different from the laparoscopic image, and controls the movements of the robotic arms using two hand controls and five foot switches: clutch, camera, focusing, monopolar (cutting and coagulating), and bipolar. The surgeon’s arms and hands can be maintained in a comfortable working position, because pressing the clutch foot switch disengages the robotic arms from the surgeon hands. This allows the surgeon to reposition the arms to an appropriate and comfortable position without moving the portion of the robotic arms connected to the patient.
Figure 25.7 The surgeon is seated at the console and is able to maintain a relaxed position for the arms and legs.
Because the surgeon is separated from the patient, there is a time delay that permits the performance of telesurgery, as long as the latency time does not exceed 150 milliseconds. The surgeon can control the movements of the robotic arms and instruments in a patient located at a different geographic location (11). Telementoring allows the mentor surgeon to guide another surgeon through a surgical procedure at a remote location by using superimposed electronic signals (telestration), direct voice commands, and control of the laparoscope via joystick and the electrosurgical instruments (12). Robotic movements are intuitive; the tip of the instrument mimics the movement of the surgeon’s hands, in contrast to the experience in laparoscopy when the movements of the tip of the instrument are counterintuitive, opposite to the movements of the surgeon’s hands.
Other technological advantages include downscaling of movements, absence of tremor, and stillness. In laparoscopy the amplitude of movement of the tip of the instrument is the same as the amplitude of the movement of the surgeon’s hands, whereas in robotics it can be downsized, resulting in increased precision. The computerized interface between surgeon hands and robotic instruments eliminates unwanted tremor, increasing precision. When placed in a specific position, such as for retracting bowel or uterus, the robotic instruments remain in place until repositioned, which can be a difference from human assistance.
The lack of tactile feedback (haptics) is a drawback for the neophyte, but the stereoscopic view rapidly compensates for it. There is a degree of resistance felt at the hand controls of the console, which, coupled with the stereoscopic view, allows the surgeon to determine the consistency of tissues, whether soft or hard, such as “to palpate” the rim of the cervical cup of a uterine manipulator, or “feel” a hard probe introduced in the rectum. The lack of tactile feedback is an advantage when operating in obese patients, because the surgeon cannot feel the resistance of the instruments being inserted through a thick abdominal wall, a difference from laparoscopy.
Another potential advantage of the robotic laparoscopy technique compared to laparoscopy without the assistance of robotics is that it may decrease the number of injuries in surgeons. There are multiple reports of laparoscopic-related surgeon morbidity syndromes, resulting from the unnatural forced position of wrists, fingers, elbows, and shoulders and the awkward standing position of the surgeon (13). A recent study revealed a lower number of surgeon injuries and lower perception of surgeon’s pain, numbness, and fatigue using robotics compared to conventional laparoscopy (14).
Instrumentation
Two of the most important differences between conventional laparoscopic instruments and robotic instruments are articulation and intuitive movements. The tip of a laparoscopic instrument is rigid and the instrument only has 4 degrees of freedom. The tip of robotic instruments are articulated with 7 degrees of freedom, reproducing the movements of the human wrist and fingers (Fig. 25.3) (EndoWrist instruments, Intuitive Surgery Inc, Sunnyvale, CA). The articulation allows the performance of complex maneuvers in small spaces; it accommodates the instrument to the correct plane of dissection (instead of forcing the tissue to the direction of the instrument as in laparoscopy), eliminates changing instruments from one port to another as in conventional laparoscopy, and facilitates suturing and intracorporeal knot tying. As indicated above, the movement of the robotic instruments is intuitive, following the movement of the surgeon hands.
Types of Instruments
Robotic instruments useful for gynecological surgery include ProGrasp forceps, PK dissecting forceps, tenaculum forceps, SutureCut needle driver, monopolar curved scissors, and monopolar cautery spatula (Fig. 25.5A-F).
Trocars
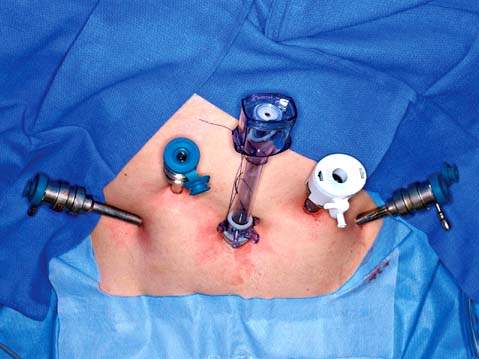
The robotic trocars are metallic and have three different types of obturators: blunt, tissue-spreading, and cutting (Fig. 25.4). The robotic trocars are placed in a different configuration than conventional laparoscopy trocars because of technical limitations imposed by the robotic arms. They are usually placed at the level of the umbilicus or higher for pelvic surgery and must be 10 cm apart from each other and from the laparoscope to prevent collision of the robotic arms (Fig. 25.8). At least one assistant trocar is necessary, which must be placed 3 to 5 cm away from any of the other trocars.
Figure 25.8 Trocar position for robotic pelvic surgery. The optical trocar is at the umbilicus. Two robotic trocars are placed 10 cm lateral to the right and left of the umbilicus, respectively. The assistant white trocar is 3 cm cranial and equidistant between the umbilical and left robotic trocar. An additional robotic trocar is 3 cm cranial and equidistant between the umbilical and the right robotic trocar.
Docking
Docking is a robotic term defined as the attachment of the robotic arms to the robotic trocars inserted in the patient. A mean docking time of about 3 minutes was reported in an initial study of 88 patients undergoing robotic hysterectomy (6). Docking times improved progressively after groups of 10 patients each.
Certification and Credentialing in Robotics
There is a mandatory training course of 2 days to obtain certification in robotics provided by the manufacturing company Intuitive, Inc (Sunnyvale, CA) in centers located in the United States, Europe, and Asia. The gynecologist learns the robotic system and logs about 8 hours of dry and animal laboratory tasks. Residents or fellows completing training programs with robotics must demonstrate proficiency with documented basic robotic training and successfully perform a minimum of 10 robotic surgeries, preferably of the same type, and obtain a letter from the program director attesting to their level of robotic proficiency. Credentialing is dependent on each hospital credentialing body and is different for each institution: while some require robotic practice in five animals and having a proctor for the first five robotic surgeries, others mandate having a proctor for only two to five robotic procedures prior to obtaining robotic privileges.
Assistant
An assistant is needed for docking, switching robotic instruments, solving robotic arm collision, retrieving small specimens, retracting tissue, and for the use of instruments not available with robotic technology, such as a vessel-sealing device and suction and irrigation. The assistant must be trained in robotics and be very knowledgeable about the procedure, because the surgeon is seated in a separate place and not scrubbed.
Teaching Robotics
The robotic teaching console allows the surgeon and the resident or fellow to each have his or her own console while having dual control. The surgeon is able to demonstrate the performance of a specific task to the trainee, or to assist the resident with one robotic arm, or prevent an imminent injury by disengaging the robotic arms from the resident’s hands, while both surgeon and resident are seated and viewing the stereoscopic image. The benefits of the teaching console were not compared to standard laparoscopic teaching to evaluate advantages. The half million dollar cost of the teaching console is a large disadvantage.
Previous laparoscopic experience was considered an advantage to learning robotics. Multiple reports show that transitioning from laparotomy to robotics is easier than transitioning from laparoscopy to robotics (15). A large number of gynecologic oncologists and gynecologists incorporated robotics into their daily practice without having incorporated laparoscopy into their practice or performing advanced laparoscopic procedures. In one study the initiation of robotics in a gynecologic oncology program reduced laparotomy from 78% to 35% in the first year of implementation and it increased the number of minimally invasive procedures from 22% performed by laparoscopy to 65% being robotic assisted (15). Thus, robotic technology is relatively easy to use, associated with a short learning curve, and comfortable for the surgeon (15–17). It is less stressful to learn robotics than laparoscopy. Reduced stress and ease of learning with robotics compared to laparoscopy was demonstrated when 16 medical students were exposed to both techniques (5).
Robotic Applications in Benign Gynecology
The robotic system offers advantages for the surgeon, but it is particularly useful in four circumstances: obese patients, long surgeries, and operations requiring extensive suturing or high precision. There is no resistance felt by the surgeon hands to the movement of the robotic instruments, regardless of the thickness of the patient’s abdominal wall. Suturing and intracorporeal knot tying are greatly facilitated by the articulation of the robotic instruments. Surgeon’s fatigue is decreased when seated for long operations. High precision and absence of tremor is useful for retroperitoneal lymphadenectomy, adhesiolysis, parametrial ureteral dissection, and accurate suturing, such as for ureteral anastomosis or reimplantation and genitourinary fistula repair. Applications of robotic technology include simple hysterectomy, myomectomy, appendectomy (in conjunction with other procedures), adnexectomy, excision of severe endometriosis, tubal anastomosis, and rectovaginal or vesicovaginal fistulas, especially those in the upper vagina (1,4). Robotic surgery is associated with a similar or shorter surgical time than conventional laparoscopy for the performance of simple hysterectomy and myomectomy, with a reduced blood loss, complication rates, and hospital stays (2,4).
Hysterectomy
Laparoscopic-assisted vaginal hysterectomy was introduced in 1989. By 2005, only 14% of hysterectomies in United States were laparoscopic (18). By contrast, 3 years after FDA approval of robotic hysterectomy in 2005, 10% of hysterectomies were performed robotically in 2008 (Intuitive, Inc).
A report on 91 patients undergoing a robotic simple hysterectomy demonstrated a mean surgical time of 127.8 minutes, blood loss of 78.6 mL, and hospital stay of 1.4 days. One enterotomy was repaired robotically. There were no conversions to laparoscopy or laparotomy. Postoperative complications included single instances of exacerbation of congestive heart failure, ileus, vaginal cuff abscess, and Clostridium difficile colitis (19). A retrospective comparison of robotic and laparoscopic hysterectomy showed a longer operating time for robotics (27 minutes) but a lower blood loss and shorter hospital stay (19). Postoperative complications were similar. The conversion rate was higher for laparoscopy compared to robotic operations (9% vs. 4%, respectively).
Myomectomy
Laparoscopic myomectomy provides less morbidity and a shorter recovery time compared to open myomectomy in two prospective randomized studies (20,21). Similarly, robotic myomectomy morbidity and recovery times appear preferable to open myomectomy. In a comparison of both techniques, robotic patients experienced less blood loss, no blood transfusions, lower postoperative complications, and shorter hospital stays. The operating time and cost were increased in the robotic group (22). In an initial series of 35 patients undergoing robotic myomectomy, the mean operating time was 230.8 minutes, mean blood loss was 169 mL, and median hospital stay was 1 day (23). Two patients (8.6%) required conversion to laparotomy, resulting from inadequate robotic instrumentation for myomectomy.
Robotic myomectomy provided comparable perioperative results to laparoscopy in two retrospective studies of patients with symptomatic myomas (24,25). No significant differences were noted between both groups for blood loss, complications, and hospital stay. The robotic operating time was longer than laparoscopy in one of the studies and no different in the other (24,25). In our comparison study, we noted differences favorable to the robotic group relative to operating time (141 vs. 166 minutes), blood loss (100 vs. 250 mL), and hospital stay over 2 days (12% vs. 23%). There were no significant differences between groups when patients were adjusted for uterine size and myoma weight. There were no differences in intra- or postoperative complications. Postmyomectomy pregnancy rates, uterine rupture, and late operative complications using robotics remain undetermined.
Adnexectomy
Numerous studies show the benefits of laparoscopy over laparotomy for patients with an adnexal mass. Robotics provides similar perioperative outcomes when compared to laparoscopy for patients with an adnexal mass. Outcomes were improved with robotics for obese patients (body mass index [BMI] ≥30) with an adnexal mass. In a comparison series of 176 patients with an adnexal mass, 85 operations were by robotics and 91 by laparoscopy (26). The operating time was 12 minutes longer for the entire robotic group (83 vs. 71 minutes), but it was similar to laparoscopy when comparing only patients with a BMI 30 or more (80 vs. 71 minutes). Blood loss was similar for both groups (39.1 vs. 41.2 mL), but lower for the robotic group when comparing only obese patients (BMI ≥30) (39 vs. 60 mL). The length of hospital stay, as measured by the number of patients staying longer than 2 days (0 vs. 3) was similar for both groups.
Tubal Reversal
Robotic tubal reversal may be preferable to laparotomy. In a prospective study comparing both techniques on patients with a previous tubal ligation, the operating time was longer using robotics (201 vs. 155 minutes), but the hospital stay (4 hours vs. 1.3 days), and recovery time to normal activities (11.1 vs. 28.1 days) were improved for robotic patients (27). Both groups had similar pregnancy rates (62.5% vs. 50%). In another retrospective case-control study of outpatient tubal anastomosis performed by robotics or minilaparotomy, perioperative outcomes were similar except for a longer operating time, increased cost, and a shorter time to patient’s recovery with robotics (28).
Appendectomy
Reported results on 107 robotic appendectomies performed in conjunction with other pelvic procedures had a mean time for appendectomy of 3.4 minutes. No perioperative complications related to appendectomy were encountered. Increased abnormal pathological findings were observed in patients with pelvic pain compared to those without pain (37% vs. 15%, respectively). Appendiceal metastases were found in 43% of patients with ovarian malignancy (29).
Sacrocolpopexy
The robotic approach to sacrocolpopexy may be better than conventional laparoscopy because of the ease of suturing and of intracorporeal knot tying. A report of feasibility experience with 80 patients demonstrated total operating time of 197.9 minutes, and most patients underwent additional procedures at the same surgical setting (30). There was a 25.4% decrease of the operating time after the first 10 surgeries. Complications were minimal. Other reported operating times are 317 and 328 minutes (31,32).
Robotic sacrocolpopexy may be preferable to laparotomy because of improved perioperative outcomes and similar anatomical results. A comparison of 73 robotic with 105 laparotomy sacrocolpopexies for vaginal and uterine prolapse revealed a longer operating time (328 vs. 225 minutes) but reduced blood loss (103 vs. 255 mL) and shorter hospital stay (1.3 vs. 2.7 days) for the robotic group. Anatomical improvement was similar for both groups as determined by pelvic organ prolapse quantification (POP-Q) C point (−9 vs. −8) (see Chapter 27) (32). Late complications, such as erosion rate and long-term anatomical results of robotic sacrocolpopexy are unknown.
Gynecologic Oncology
Studies show the feasibility of robotics for the treatment of patients with cervical, endometrial, tubal, and ovarian cancer, with apparent similar or better perioperative outcomes, compared to conventional laparoscopy and with improved outcomes compared to laparotomy (33–39). A survey of Society of Gynecologic Oncologists members indicated 24% are regular users of robotics and 66% believed their use would increase (33).
Endometrial Cancer
Laparoscopy provides similar or longer operative times, less blood loss, fewer postoperative complications, shorter hospital stay and recovery times, with similar recurrence and survival rates compared to laparotomy (34–39). Robotic technology may be preferable to conventional laparoscopy in selected patients for surgery for endometrial cancer, especially in morbidly obese patients. In expert hands, the use of minimally invasive techniques—laparoscopy and robotics—may facilitate the operations.
Studies comparing robotics to laparotomy suggest improved perioperative outcomes for robotics. Operative times are similar or longer, blood loss and hospital stay are reduced, the number of lymph nodes is comparable or higher, and postoperative complications are similar or decreased with robotics (15–17,40–44). Studies comparing robotics with conventional laparoscopy for endometrial cancer show similar or superior results for robotics. Some showed reduced blood loss, shorter hospital stay, increased lymph node yield, and lower morbidity with robotics compared to conventional laparoscopy (41). Others reported longer operating time but reduced blood loss, lower transfusion rate, lower conversion rate to laparotomy, and reduced hospital stay, even though the robotic patients had a higher BMI compared to laparoscopy (44). Robotics, as with laparoscopy, resulted in a shorter recovery time for endometrial cancer patients compared to laparotomy (40,45).
In an initial review of 38 patients with robotics operations, the perioperative outcomes were similar to patients treated by laparoscopy (n = 22). Results were similar to patients treated by laparotomy (n = 16) other than a larger blood loss and longer hospital stay for this group of patients. The operating times were similar for the three groups, blood loss was lower for the robotic and laparoscopy groups (283, 222, and 517 mL), and length of hospital stay was shorter for the robotic and laparoscopy groups (2, 2.5, and 6 days). There was no difference relative to the number of lymph nodes removed (18.4, 26.3, and 18.4, for robotics, laparoscopy, and laparotomy, respectively), number of lymph node metastases, positive cytological findings, or tumor recurrence. Postoperative complications were comparable among the three groups.
Boggess et al. compared 103 robotic patients with 81 laparoscopy patients and 138 laparotomy patients (41). The operating times for robotic and laparoscopy procedures were similar, but longer than laparotomy (191.2 minutes, 213.4 minutes, and 146.5 minutes, respectively). The blood loss, hospital stay, and number of nodes were all improved for the robotic group. Bell et al. compared a group of 40 robotic patients with 30 patients operated by laparoscopy and 40 patients operated by laparotomy (40). Robotic and laparoscopy operating times were comparable (184 minutes and 171 minutes, respectively) and longer than laparotomy (108.6 minutes). Blood loss was similar to laparoscopy, and both were lower than the laparotomy group. The number of lymph nodes removed was similar among the three groups. Postoperative complications were lower in the robotic group compared to the other two groups (7.5%, 27.5%, and 20%, respectively).
Another study evaluated the results of robotics and conventional laparoscopy for obese and morbidly obese patients (BMI 30–60) with endometrial cancer (42). Robotics was found to be preferable to the laparoscopic approach because of a shorter operative time, reduced blood loss, increased number of lymph nodes removed, and shorter hospital stay. The benefits of robotics for obese patients was noted with simple robotic hysterectomy, where the operating time remained unchanged in spite of the patient’s increased BMI (6). This may result from the absent awareness of the resistance of the robotic instruments being inserted through a thick abdominal wall because of the lack of tactile feedback.
The application of robotics for endometrial cancer can result in improvements of the perioperative outcomes (43). The operating time decreased by 47 minutes, vaginal cuff delayed healing decreased from 16% to 0%, and the number of pelvic and aortic lymphadenectomies and number of nodes retrieved increased per quarter analyzed from the initiation of robotics.
The increased costs of this technology is a criticism of robotics. Because cost is tied to frequency of use, when used on a regular basis the cost becomes similar to laparoscopy and less expensive than laparotomy as a result of shorter hospital stay. A comparison study of costs for robotics, conventional laparoscopy, and laparotomy for endometrial cancer showed a significantly reduced cost for robotics and laparoscopy compared to laparotomy and comparable costs for robotics and laparoscopy (40).
Cervical Cancer
Early Cervical Cancer
Robotic and laparoscopic radical hysterectomy may provide patient benefits over a laparotomy approach relative to blood loss, blood transfusions, and length of hospital stay. Operating times and postoperative complications are lower or similar (46–52). Recurrence and survival rates remain unchanged when comparing the results of both techniques (46–48,51,52). A prospective randomized trial is under way comparing laparoscopic or robotic radical hysterectomy with laparotomy (53).
The surgical technique for robotic radical hysterectomy is described elsewhere (54). A rapid learning curve is noted with robotic radical hysterectomy, with a 44-minute reduction in operating time after the first 34 procedures in association with a low complication rate and high nodal count (55).
In several retrospective studies comparing laparotomy with robotic radical hysterectomy, there was a shorter, similar, or longer operating time; reduced blood loss; shorter hospital stay; lower, similar, or higher number of lymph nodes removed; and similar rate of postoperative complications (49,56–58). In comparison with laparoscopy, robotics is associated with a shorter, similar, or longer operating time; reduced blood loss; shorter hospital stay; similar or lower postoperative complications; and comparable or higher number of nodes (49,50,57).
Tumor recurrence does not appear increased with robotics compared to laparoscopy. At a mean length of follow-up of 31.1 months (range, 10 to 50 months), none of the patients in the robotic group experienced recurrence, and there was no difference with the laparoscopy group (50).
Other Applications of Robotics for Cervical Cancer
Radical parametrectomy is a surgical option for patients with an undiagnosed invasive cervical carcinoma discovered on a simple hysterectomy specimen with negative margins and with no gross visible disease. The feasibility of robotic radical parametrectomy was reported by Ramirez et al. in five patients with acceptable complications (59). There are no comparison studies of robotic radical parametrectomy with laparoscopy or laparotomy.
Radical trachelectomy for cancer of the residual cervical stump is preferable to pelvic irradiation in some patients because of the increased risk of bowel complications from intestinal adhesions to the remaining cervix. The laparoscopic approach is previously reported (60). The use of robotic radical trachelectomy in a patient with a “cut-through” endometrial cancer discovered in the subtotal hysterectomy specimen was reported (61). Radical trachelectomy is an alternative to radical hysterectomy for cervical cancer in young patients desiring fertility and with a tumor size of 2 cm or less, no lymphatic invasion or nodal metastases, and potential for preservation of the upper portion of the cervix. The feasibility of robotic radical trachelectomy was demonstrated in two patients with early stage cervical cancer (62). The operative times were longer (387 and 358 minutes), because of the novelty of the procedure and the required waiting time for the frozen section. No intra- or postoperative complications were observed. There are no comparison studies of robotic radical trachelectomy with laparoscopy or laparotomy. A review of eight reported patients up to May 2009 showed a median operating time of 339 minutes, median blood loss of 62.5 mL, no intraoperative complications, no blood transfusions, no conversions to laparotomy, and a median length of hospital stay of 1.5 days (63). The median number of pelvic lymph nodes removed was 20.
Retroperitoneal Lymphadenectomy for Advanced Cervical Cancer
The laparoscopic approach to pelvic and aortic lymphadenectomy prior to chemoirradiation for patients with advanced cervical cancer is well documented, both transperitoneal and extraperitoneal (64–69). There are many studies discussing robotic pelvic and aortic lymphadenectomy included in the treatment of patients with different types of gynecologic malignancies, including cervical, endometrial, and ovarian malignancies (16,49,50,55–58,70,71).
A novel technique and the results of robotic transperitoneal infrarenal aortic lymphadenectomy in 33 patients with different gynecologic cancers was reported (72). The requirement of this technique was the placement of the robotic column at the patient’s head, which mandated the rotation of the operating table 180 degrees after completion of the robotic pelvic operation and the insertion of additional trocars in the lower pelvis (72). The mean console time was 42 minutes, the mean number of nodes 12.9, and the mean number of positive nodes 2.6. There was one conversion to laparotomy caused by bleeding. These results are comparable to those reported with laparoscopic aortic lymphadenectomy. Placing the robotic column at the patient’s head and inserting additional trocars in the lower pelvis were requirements to remove the infrarenal aortic nodes with robotics. Previous attempts to remove the left infrarenal aortic lymph nodes in a frozen cadaver were unsuccessful when the robotic column was stationed at the level of the patient’s feet.
The feasibility of the extraperitoneal robotic approach to aortic lymphadenectomy was reported (73,74). The development of the robotic extraperitoneal technique in frozen cadavers, successfully applied to a patient with cervical cancer stage IB2 presenting with enlarged left aortic lymph nodes, is reported (73). It was noted that appropriate placement of the robotic trocars and the robotic column is necessary to prevent robotic arm collision and to reach the different aortic lymph nodal areas. The operating, docking, and console times were 103, 3.5, and 49 minutes, respectively. The blood loss was 30 mL. Selective removal of five enlarged aortic lymph nodes revealed no evidence of metastases.
Another study by Vergote et al. reported the development of their technique for inframesenteric aortic lymphadenectomy in five patients (74). All console times were less than 1 hour. The operating times and number of aortic lymph nodes in both studies are within the range of those reported with the laparoscopic approach. Both studies concluded that the robotic approach was technically easier than the conventional laparoscopy procedure (73,74).
Robotic Pelvic Exenteration for Recurrent Cervical Cancer
Robotic anterior pelvic exenteration was described in three patients with recurrent cervical cancer (75). The operating times (range, 480 to 600 minutes) are longer than for laparotomy, but blood loss (range, 200 to 500 mL) is markedly lower. The length of hospital stay was similar or longer than by laparotomy (range, 25 to 53 days). Patients required a pararectal incision (10 cm) for the pouch construction, and one patient with vaginal reconstruction had a perineal phase. Whether the robotic approach to pelvic exenteration will become preferable to laparotomy is unknown.
Ovarian Cancer
Surgery for ovarian cancer requires access to all four abdominal quadrants. The ability to operate in all four quadrants using robotics can only be achieved by rotating the operating table and positioning the robotic column at the patient’s head. In this position it is possible to excise the aortic lymph nodes to the level of the renal vessels and to perform resection of upper abdominal metastases, including small bowel, diaphragm, liver, and supracolic omental disease. Initial experience with 21 patients with ovarian cancer who underwent operations by robotics was reported (76). Included were 12 primary, 4 interval, and 5 secondary cytoreductive surgeries for ovarian cancer. In addition to primary tumor excision, major procedures included modified posterior pelvic exenteration, rectosigmoid resection, small bowel resection, diaphragm resection, and resection of liver metastases. The operating times had a wide range (103 to 454 minutes) resulting from the extent of procedures. Blood loss was low (25 to 300 mL). Postoperative complications were low but increased with the number of procedures.
There are reports of robotics for ovarian cancer consisting of an occasional patient included within a series of patients with different types of gynecologic cancers and with no comparison to laparoscopy or laparotomy (77–81). Robotics may be useful for selected patients with ovarian cancer. Because of the need for operating table rotation and the insertion of additional trocars, it may not become widely accepted. The use of the surgical robotic system can be advocated in the case of isolated recurrences of ovarian cancer, such as in the pelvis, or in the upper abdomen, diaphragm, liver, and spleen. Isolated left liver metastases were removed successfully in three patients with recurrent ovarian cancer with no complications (82).
In conclusion, robotics and laparoscopy have advantages compared to laparotomy for selected patients with cervical, endometrial and early ovarian cancer. Although prospective randomized trials are desirable, the retrospective evidence supports the selected use of minimally invasive surgery for endometrial and cervical cancer patients and selected patients with primary or recurrent ovarian cancer.
Complications Unique to Robotics
Because there is no tactile feedback, the robotic arms can injure any organ not under visual control. The potential for injury is higher with the fourth retracting arm when out of the visual field and repositioned without visual control. Constant visual control of the robotic instrument position is mandatory for the prevention of injuries. Collision of the robotic arms is felt as an unwanted resistance, which is realized when the instruments are brought under view.
Constant pressure of the robotic arms on the patient thighs or arms may result in injury. This can be prevented during positioning of the trocars and robotic arms. A rapid move by the surgeon on any robotic instrument may result in hitting the assistant near the operating table with the robotic arm. Moving a robotic arm by anyone other than the surgeon may result in patient internal injury.
Because the robotic trocars are fastened to the robotic arms, and the arms are fixed to the robotic column, any sudden loss of the pneumoperitoneum may result in the instruments being pulled off the anterior abdominal wall as it becomes flattened. Similarly, any sliding of the patient on the operating table as a result of the Trendelenburg position will result in pressure at the trocars site and potential injury or increased postoperative trocar site pain. The robotic column must be positioned at the level of the patient’s feet or lateral to the legs in order to prevent access to the vagina, rectum, or urethra.
Disadvantages of Robotics
The present robotic system has limited surgical field reach. It allows for pelvic or abdominal surgery, but not both, unless the robotic column is repositioned from the patient’s feet to the patient’s head. This repositioning increases surgical time and requires the insertion of additional trocars.
The available robotic instrumentation is appropriate for gynecological surgery, but there is no vessel sealing cutting device or suction-irrigation device. An assistant is necessary, at the surgical site away from the surgeon, and therefore, all orders or corrections are verbal.
Although the grasping pressure of the robotic instruments is high and can damage tissue, tissue traction is not as efficient as with conventional laparoscopic instruments. Because there is no tactile feedback when grasping a tissue, there is only visual control of the traction exerted upon any structure. The lack of tactile feedback is an advantage in obese patients (see above technology), but can be a disadvantage when the instruments are not in the visual field.
Cost is a common reason why many institutions have delayed the initiation of robotic programs. The initial purchase of the robotic system is high, about $1.5 million, with an additional annual maintenance fee, and all robotic instruments become functionless after ten uses, so they are disposed.
The robotic column and its arms are bulky, with a total weight of 1,200 lb. An operating room must have adequate space, preferably about 600 square feet. There must be a dedicated team for robotic surgery and preferably a specific team for each surgical specialty. The surgical assistant must be versed in robotic technology and be able to provide the required maneuvers without direct interaction because the surgeon is away at a console and not in a surgical sterile condition.
References
1. Moorthy K, Munz Y, Dosis A, et al. Dexterity enhancement with robotic surgery. Surg Endosc 2004;18:790–795.
2. Prasad SM, Prasad SM, Maniar HS, et al. Surgical robotics: impact of motion scaling on task performance. J Am Coll Surg 2004;199:863–868.
3. Sarle R, Tewari A, Shrivastava A, et al. Surgical robotics and laparoscopic training drills. J Endourol 2004;18:63–67.
4. Yohannes P, Rotariu P, Pinto P, et al. Comparison of robotic versus laparoscopic skills: is there a difference in the learning curve? Urology 2002;60:39–45.
5. van der Schatte Olivier RH, Van’t Hullenaar CD, Ruurda JP, et al. Ergonomics, user comfort, and performance in standard and robot-assisted laparoscopic surgery. Surg Endosc 2009;23:1365–1371.
6. Kho RM, Hilger WS, Hentz JG, et al. Robotic hysterectomy: technique and initial outcomes. Am J Obstet Gynecol 2007;197:113.e111–e114.
7. Kreiker GL, Bertoldi A, Larcher JS, et al. Prospective evaluation of the learning curve of laparoscopic-assisted vaginal hysterectomy in a university hospital. J Am Assoc Gynecol Laparosc 2004;11:229–235.
8. Lenihan JP, Jr., Kovanda C, Seshadri-Kreaden U. What is the learning curve for robotic assisted gynecologic surgery? J Minim Invasive Gynecol 2008t;15:589–594.
9. Pitter MC, Anderson P, Blissett A, et al. Robotic-assisted gynaecological surgery-establishing training criteria; minimizing operative time and blood loss. Int J Med Robot 2008;4:114–120.
10. Seamon LG, Fowler JM, Richardson DL, et al. A detailed analysis of the learning curve: robotic hysterectomy and pelvic-aortic lymphadenectomy for endometrial cancer. Gynecol Oncol 2009;114:162–167.
11. Anvari M, McKinley C, Stein H. Establishment of the world’s first telerobotic remote surgical service: for provision of advanced laparoscopic surgery in a rural community. Ann Surg 2005;241:460–464.
12. Rodrigues Netto N Jr, Mitre AI, Lima SV, Fugita OE, et al. Telementoring between Brazil and the United States: initial experience. J Endourol 2003;17:217–220.
13. Reyes DA, Tang B, Cuschieri A. Minimal access surgery (MAS)-related surgeon morbidity syndromes. Surg Endosc 2006;20:1–13.
14. Gofrit ON, Mikahail AA, Zorn KC, et al. Surgeons’ perceptions and injuries during and after urologic laparoscopic surgery. Urology 2008;71:404–407.
15. Peiretti M, Zanagnolo V, Bocciolone L, et al. Robotic surgery: changing the surgical approach for endometrial cancer in a referral cancer center. J Minim Invasive Gynecol 2009;16:427–431.
16. DeNardis SA, Holloway RW, Bigsby GEt, et al. Robotically assisted laparoscopic hysterectomy versus total abdominal hysterectomy and lymphadenectomy for endometrial cancer. Gynecol Oncol 2008;111:412–417.
17. Veljovich DS, Paley PJ, Drescher CW, et al. Robotic surgery in gynecologic oncology: program initiation and outcomes after the first year with comparison with laparotomy for endometrial cancer staging. Am J Obstet Gynecol 2008;198:679.e671–e679.
18. Jacoby VL, Autry A, Jacobson G, et al. Nationwide use of laparoscopic hysterectomy compared with abdominal and vaginal approaches. Obstet Gynecol 2009;114:1041–1048.
19. Payne TN, Dauterive FR. A comparison of total laparoscopic hysterectomy to robotically assisted hysterectomy: surgical outcomes in a community practice. J Minim Invasive Gynecol 2008;15:286–291.
20. Mais V, Ajossa S, Guerriero S, et al. Laparoscopic versus abdominal myomectomy: a prospective, randomized trial to evaluate benefits in early outcome. Am J Obstet Gynecol 1996;174:654–658.
21. Seracchioli R, Rossi S, Govoni F, et al. Fertility and obstetric outcome after laparoscopic myomectomy of large myomata: a randomized comparison with abdominal myomectomy. Hum Reprod 2000;15:2663–2668.
22. Advincula AP, Xu X, Goudeau St, et al. Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparison of short-term surgical outcomes and immediate costs. J Minim Invasive Gynecol 2007;14:698–705.
23. Advincula AP, Song A, Burke W, et al. Preliminary experience with robot-assisted laparoscopic myomectomy. J Am Assoc Gynecol Laparosc 2004;11:511–518.
24. Bedient CE, Magrina JF, Noble BN, et al. Comparison of robotic and laparoscopic myomectomy. Am J Obstet Gynecol 2009;201:566.e561–e565.
25. Nezhat C, Lavie O, Hsu S, et al. Robotic-assisted laparoscopic myomectomy compared with standard laparoscopic myomectomy—a retrospective matched control study. Fertil Steril 2009;91:556–569.
26. Magrina JF, Espada M, Munoz R, et al. Robotic adnexectomy compared with laparoscopy for adnexal mass. Obstet Gynecol 2009;114:581–584.
27. Dharia Patel SP, Steinkampf MP, Whitten SJ, et al. Robotic tubal anastomosis: surgical technique and cost effectiveness. Fertil Steril 2008;90:1175–1179.
28. Rodgers AK, Goldberg JM, Hammel JP, et al. Tubal anastomosis by robotic compared with outpatient minilaparotomy. Obstet Gynecol 2007;109:1375–1380.
29. Akl MN, Magrina JF, Kho RM, et al. Robotic appendectomy in gynaecological surgery: technique and pathological findings. Int J Med Robot 2008;4:210–213.
30. Akl MN, Long JB, Giles DL, et al. Robotic-assisted sacrocolpopexy: technique and learning curve. Surg Endosc 2009;23:2390–2394.
31. Daneshgari F, Kefer JC, Moore C, et al. Robotic abdominal sacrocolpopexy/sacrouteropexy repair of advanced female pelvic organ prolapse (POP): utilizing POP-quantification-based staging and outcomes. BJU Int 2007;100:875–879.
32. Visco AG, Advincula AP. Robotic gynecologic surgery. Obstet Gynecol 2008;112:1369–1384.
33. Mabrouk M, Frumovitz M, Greer M, et al. Trends in laparoscopic and robotic surgery among gynecologic oncologists: a survey update. Gynecol Oncol 2009;112:501–505.
34. Magrina JF, Weaver AL. Laparoscopic treatment of endometrial cancer: five-year recurrence and survival rates. Eur J Gynaecol Oncol 2004;25:439–441.
35. Malur S, Possover M, Michels W, et al. Laparoscopic-assisted vaginal versus abdominal surgery in patients with endometrial cancer—a prospective randomized trial. Gynecol Oncol 2001;80:239–244.
36. Nezhat F, Yadav J, Rahaman J, et al. Analysis of survival after laparoscopic management of endometrial cancer. J Minim Invasive Gynecol 2008;15:181–187.
37. Cho YH, Kim DY, Kim JH, et al. Laparoscopic management of early uterine cancer: 10-year experience in Asian Medical Center. Gynecol Oncol 2007;106:585–590.
38. Magrina JF. Outcomes of laparoscopic treatment for endometrial cancer. Curr Opin Obstet Gynecol 2005;17:343–346.
39. Magrina JF, Mutone NF, Weaver AL, et al. Laparoscopic lymphadenectomy and vaginal or laparoscopic hysterectomy with bilateral salpingo-oophorectomy for endometrial cancer: morbidity and survival. Am J Obstet Gynecol 1999;181:376–381.
40. Bell MC, Torgerson J, Seshadri-Kreaden U, et al. Comparison of outcomes and cost for endometrial cancer staging via traditional laparotomy, standard laparoscopy and robotic techniques. Gynecol Oncol 2008;111:407–411.
41. Boggess JF, Gehrig PA, Cantrell L, et al. A comparative study of 3 surgical methods for hysterectomy with staging for endometrial cancer: robotic assistance, laparoscopy, laparotomy. Am J Obstet Gynecol 2008;199:360.e361–e369.
42. Gehrig PA, Cantrell LA, Shafer A, et al. What is the optimal minimally invasive surgical procedure for endometrial cancer staging in the obese and morbidly obese woman? Gynecol Oncol 2008;111:41–45.
43. Holloway RW, Ahmad S, DeNardis SA, et al. Robotic-assisted laparoscopic hysterectomy and lymphadenectomy for endometrial cancer: analysis of surgical performance. Gynecol Oncol 2009;115:447–452.
44. Seamon LG, Cohn DE, Henretta MS, et al. Minimally invasive comprehensive surgical staging for endometrial cancer: robotics or laparoscopy? Gynecol Oncol 2009;113:36–41.
45. Zullo F, Palomba S, Russo T, et al. A prospective randomized comparison between laparoscopic and laparotomic approaches in women with early stage endometrial cancer: a focus on the quality of life. Am J Obstet Gynecol 2005;193:1344–1352.
46. Frumovitz M, dos Reis R, Sun CC, et al. Comparison of total laparoscopic and abdominal radical hysterectomy for patients with early-stage cervical cancer. Obstet Gynecol 2007;110:96–102.
47. Holloway RW, Finkler NJ, Pikaart DP, et al. Comparison of total laparoscopic and abdominal radical hysterectomy for patients with early-stage cervical cancer. Obstet Gynecol 2007;110:1174–1175.
48. Magrina JF. Robotic surgery in gynecology. Eur J Gynaecol Oncol 2007;28:77–82.
49. Magrina JF, Kho RM, Weaver AL, et al. Robotic radical hysterectomy: comparison with laparoscopy and laparotomy. Gynecol Oncol 2008;109:86–91.
50. Nezhat FR, Datta MS, Liu C, et al. Robotic radical hysterectomy versus total laparoscopic radical hysterectomy with pelvic lymphadenectomy for treatment of early cervical cancer. JSLS 2008;12:227–237.
51. Puntambekar SP, Palep RJ, Puntambekar SS, et al. Laparoscopic total radical hysterectomy by the Pune technique: our experience of 248 cases. J Minim Invasive Gynecol 2007;14:682–689.
52. Zakashansky K, Lerner DL. Total laparoscopic radical hysterectomy for the treatment of cervical cancer. J Minim Invasive Gynecol 2008;15:387–388.
53. Obermair A, Gebski V, Frumovitz M, et al. A phase III randomized clinical trial comparing laparoscopic or robotic radical hysterectomy with abdominal radical hysterectomy in patients with early stage cervical cancer. J Minim Invasive Gynecol 2008;15:584–588.
54. Magrina JF, Kho R, Magtibay PM. Robotic radical hysterectomy: technical aspects. Gynecol Oncol 2009;113:28–31.
55. Persson J, Reynisson P, Borgfeldt C, et al. Robot assisted laparoscopic radical hysterectomy and pelvic lymphadenectomy with short and long term morbidity data. Gynecol Oncol 2009;113:185–190.
56. Boggess JF, Gehrig PA, Cantrell L, et al. A case-control study of robot-assisted type III radical hysterectomy with pelvic lymph node dissection compared with open radical hysterectomy. Am J Obstet Gynecol 2008;199:357.e351–e357.
57. Estape R, Lambrou N, Diaz R, et al. A case matched analysis of robotic radical hysterectomy with lymphadenectomy compared with laparoscopy and laparotomy. Gynecol Oncol 2009;113:357–361.
58. Maggioni A, Minig L, Zanagnolo V, et al. Robotic approach for cervical cancer: comparison with laparotomy: a case control study. Gynecol Oncol 2009;115:60–64.
59. Ramirez PT, Schmeler KM, Wolf JK, et al. Robotic radical parametrectomy and pelvic lymphadenectomy in patients with invasive cervical cancer. Gynecol Oncol 2008;111:18–21.
60. Diaz-Feijoo B, Gil-Moreno A, Puig O, et al. Total laparoscopic radical trachelectomy with intraoperative sentinel node identification for early cervical stump cancer. J Minim Invasive Gynecol 2005;12:522–524.
61. Zanagnolo V, Magrina JF. Robotic radical trachelectomy after supracervical hysterectomy for cut-through endometrial adenocarcinoma stage IIB: a case report. J Minim Invasive Gynecol 2009;16:655–657.
62. Persson J, Kannisto P, Bossmar T. Robot-assisted abdominal laparoscopic radical trachelectomy. Gynecol Oncol 2008;111:564–567.
63. Ramirez PT, Schmeler KM, Malpica A, et al. Safety and feasibility of robotic radical trachelectomy in patients with early-stage cervical cancer. Gynecol Oncol 2010;116:512–515.
64. Burnett AF, O’Meara AT, Bahador A, et al. Extraperitoneal laparoscopic lymph node staging: the University of Southern California experience. Gynecol Oncol 2004;95:189–192.
65. Gil-Moreno A, Franco-Camps S, Diaz-Feijoo B, et al. Usefulness of extraperitoneal laparoscopic paraaortic lymphadenectomy for lymph node recurrence in gynecologic malignancy. Acta Obstet Gynecol Scand 2008;87:723–730.
66. Kehoe SM, Abu-Rustum NR. Transperitoneal laparoscopic pelvic and paraaortic lymphadenectomy in gynecologic cancers. Curr Treat Options Oncol 2006;7:93–101.
67. Marnitz S, Kohler C, Roth C, et al. Is there a benefit of pretreatment laparoscopic transperitoneal surgical staging in patients with advanced cervical cancer? Gynecol Oncol 2005;99:536–544.
68. Querleu D, Dargent D, Ansquer Y, et al. Extraperitoneal endosurgical aortic and common iliac dissection in the staging of bulky or advanced cervical carcinomas. Cancer 2000;88:1883–1891.
69. Tillmanns T, Lowe MP. Safety, feasibility, and costs of outpatient laparoscopic extraperitoneal aortic nodal dissection for locally advanced cervical carcinoma. Gynecol Oncol 2007;106:370–374.
70. Fanning J, Fenton B, Purohit M. Robotic radical hysterectomy. Am J Obstet Gynecol 2008;198:649.e641–e644.
71. Kim YT, Kim SW, Hyung WJ, et al. Robotic radical hysterectomy with pelvic lymphadenectomy for cervical carcinoma: a pilot study. Gynecol Oncol 2008;108:312–316.
72. Magrina JF, Long JB, Kho RM, et al. Robotic transperitoneal infrarenal aortic lymphadenectomy: technique and results. Int J Gynecol Cancer 2010;20:184–187.
73. Magrina JF, Kho R, Montero RP, et al. Robotic extraperitoneal aortic lymphadenectomy: development of a technique. Gynecol Oncol 2009;113:32–35.
74. Vergote I, Pouseele B, Van Gorp T, et al. Robotic retroperitoneal lower para-aortic lymphadenectomy in cervical carcinoma: first report on the technique used in 5 patients. Acta Obstet Gynecol Scand 2008;87:783–787.
75. Lambaudie E, Narducci F, Leblanc E, et al. Robotically-assisted laparoscopic anterior pelvic exenteration for recurrent cervical cancer: report of three first cases. Gynecol Oncol 2010;116:582–583.
76. Bandera CA, Magrina JF. Robotic surgery in gynecologic oncology. Curr Opin Obstet Gynecol 2009;21:25–30.
77. Diaz-Arrastia C, Jurnalov C, Gomez G, et al. Laparoscopic hysterectomy using a computer-enhanced surgical robot. Surg Endosc 2002;16:1271–1273.
78. Field JB, Benoit MF, Dinh TA, et al. Computer-enhanced robotic surgery in gynecologic oncology. Surg Endosc 2007;21:244–246.
79. Lambaudie E, Houvenaeghel G, Walz J, et al. Robot-assisted laparoscopy in gynecologic oncology. Surg Endosc 2008;22:2743–2747.
80. Reynolds RK, Burke WM, Advincula AP. Preliminary experience with robot-assisted laparoscopic staging of gynecologic malignancies. JSLS 2005;9:149–158.
81. van Dam PA, van Dam PJ, Verkinderen L, et al. Robotic-assisted laparoscopic cytoreductive surgery for lobular carcinoma of the breast metastatic to the ovaries. J Minim Invasive Gynecol 2007;14:746–749.
82. Choi SB, Park JS, Kim JK, et al. Early experiences of robotic-assisted laparoscopic liver resection. Yonsei Med J 2008;49:632–638.
< div class='tao-gold-member'>



