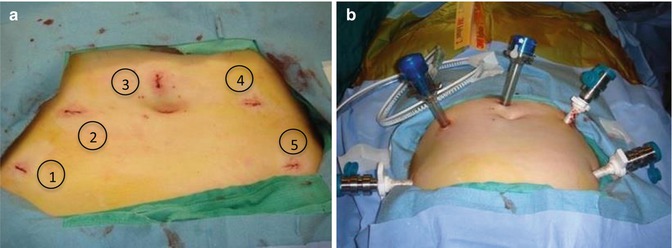
Fig. 12.2
Standard approach to robotic trocar placement. (a) Positioning of trocar sites: (1) 8 mm robotic trocar with versastep sleeve, (2) 12 mm assistant trocar, (3) 12 mm endoscope trocar, (4) 8 mm robotic trocar with versastep sleeve, (5) 8 mm robotic trocar with versastep sleeve. (b) Insufflated abdomen with robotic trocars in place (Courtesy of Karen Wang, MD)
Patients with large intramural myomas >10 cm in diameter may be best treated with hybrid robotic myomectomy technique (Quaas et al. 2010). With this approach, enucleation of myoma is performed by laparoscopy, while suturing is done robotically. Major benefits of the hybrid approach in management of large fibroids include preservation of tactile feedback during separation of large mass from the delicate surrounding tissues; the ability to use a rigid tenaculum needed to exert significant pull force during enucleation; and easy access and navigation through all four pelvic quadrants, which may be limited during the robotic procedure.
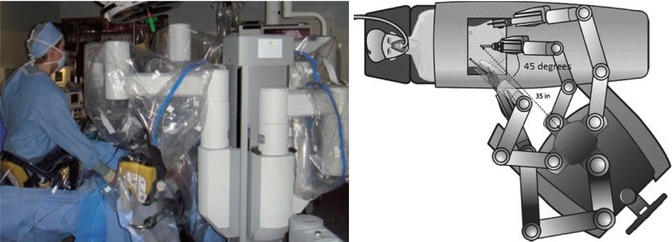
Regardless of timing, docking of robotic system can be done in various ways. One traditional approach involves positioning of the robot between the patient legs (Fig. 12.3). Vaginal access in this midline approach is limited which makes uterine manipulation difficult. More recently, side docking was developed and successfully adopted for the majority of robotic procedures (Einarsson et al. 2011b). With side docking, the robot is positioned at the left or right patient’s knee at a 45° angle from the lower torso (Fig. 12.4). Parallel docking similarly positions the robot at the patient’s side with the robot parallel to the operative table (Silverman et al. 2012) (Fig. 12.5). Such side-access set ups allow for significantly improved vaginal access and ability to use and adjust uterine manipulator intraoperatively.
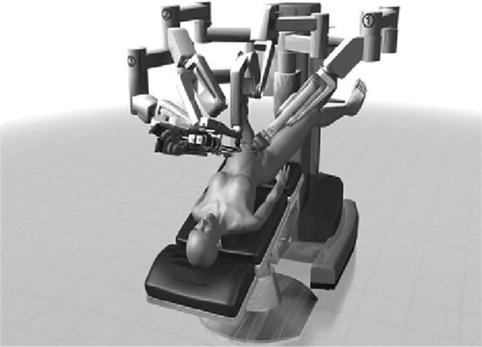

Fig. 12.3
Traditional docking of the robot between the patient’s legs
Several approaches to blood loss control during myomectomy have been described, including tourniquet application, uterine vessel clipping, vasopressin injection, and others. While approved by the US Food and Drug Administration, vasopressin use is not permitted in several European countries. Vasopressin, as well as misoprostol, bupivacaine plus epinephrine, tranexamic acid, gelatin–thrombin matrix, pericervical tourniquet, and mesna, but not oxytocin or morcellation, were found to significantly reduce bleeding during myomectomy (Kongnyuy and Wiysonge 2011). Clipping of uterine vessels is another method shown to effectively reduce blood loss during the procedure (Sinha et al. 2004; Vercellino et al. 2012; Rosen et al. 2009).
Following the appropriate measures to ensure intraoperative hemostasis, the uterine incision is made in a longitudinal or transverse manner (Quaas et al. 2010). The approach to myoma enucleation is similar to one employed during an open procedure, with robotic tenaculum, robotic bipolar coagulator, and ultrasonic or monopolar energy employed to assist in this process (Fig. 12.6a–h). The bedside assistant can place additional traction on myoma(s).
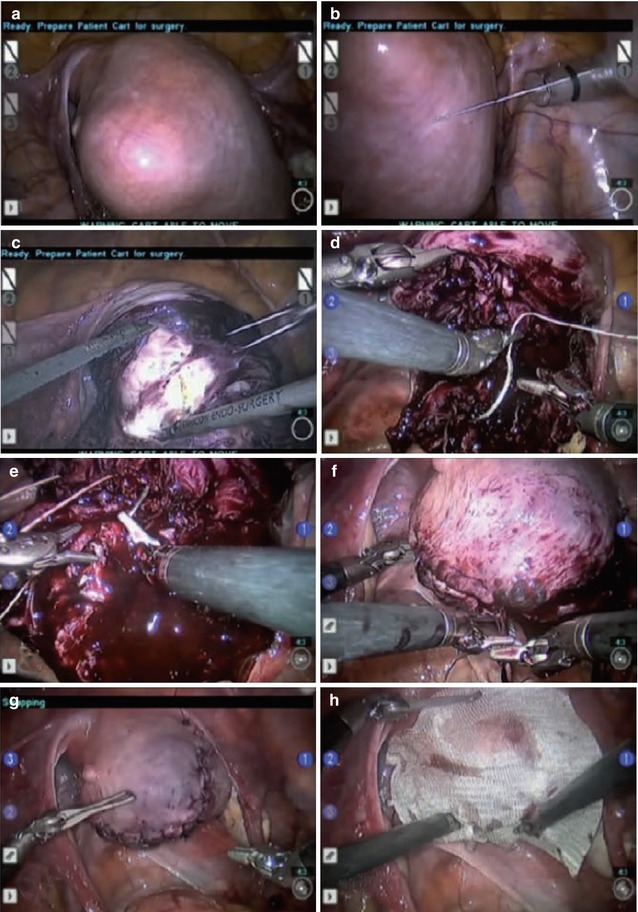

Fig. 12.6
Technique of robotic myomectomy. (a, b) After the fibroid location has been exactly determined, a dilute concentration of vasopressin is injected into the myometrium surrounding the myoma. (c) Using the robotic harmonic shears, a hysterotomy is made over the myoma. (d–g) A multilayer closure is performed employing sutures and suturing techniques that are identical to those of an open myomectomy. (h) An adhesion barrier may be placed onto the closed hysterotomy to prevent future scar tissue formation (From Quaas et al. 2010)
Following removal, the myoma is placed in the posterior cul-de-sac or upper quadrant(s) to allow unobstructed access to the uterus; it is later morcellated. Whether morcellating with a power morcellotor device or via minilaparotomy, efforts should be made to contain all tissue fragments and avoid tissue dissemination during this process. It is also important to keep track of the number of fibroids extracted to ensure that all are removed at the end of the procedure.
The closure of the myometrial defect is best performed in a multilayer fashion in order to minimize the risk of uterine rupture (Parker et al. 2010). Other factors to consider include the judicious use of electrosurgery, CO2 pneumoperitoneum effect on wound healing, and individual wound healing characteristics, including thinning myometrium (Parker et al. 2010). Successful application of the bidirectional barbed suture in laparoscopic myomectomy was previously tested and described (Greenberg and Einarsson 2008). In our practice, QuillTM (Angiotech Pharmaceuticals, Inc., Vancouver, BC, Canada), V-lockTM (Covidien, Mansfield, MA) or STRATAFIXTM (Ethicon, Somerville NJ) have been successfully utilized, and their choice depends on surgeon’s preference. The use of bidirectional suture decreases operative time and could be one of the key reasons behind the longer operating times associated with robotic myomectomy in a recent comparison of the two minimally invasive techniques at our institution (Gargiulo et al. 2012a).
With the advancements in the field of minimally invasive surgery, additional approaches to laparoscopic myomectomy are now available. A single port laparoscopic myomectomy technique has been successfully attempted in the past (Einarsson 2010), and more recently, applied to robotics (Gargiulo et al. 2012b) (Fig. 12.7). Although safe and reproducible with outcomes comparable to conventional robotic myomectomy, robot-assisted single-incision laparoscopic approach was found to be associated with additional technical challenges typical for single incision surgery, such as crowding of instruments, loss of optimum instrument triangulation, and inability to use all three robotic arms. In particular, partial loss of dexterity was noted by authors due to crowding of 8 mm instruments.
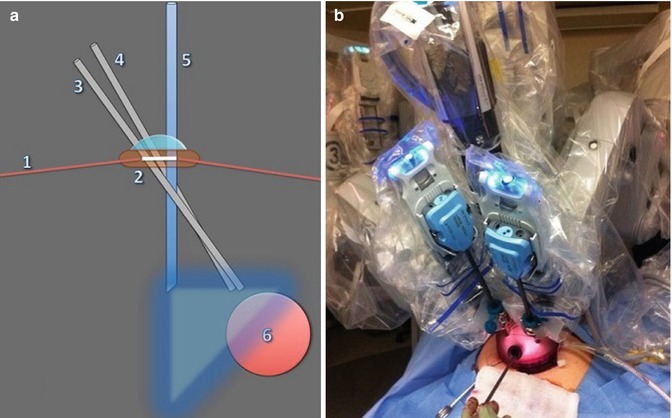

Fig. 12.7
Single port robotic myomectomy: schematic (a) and live (b) set up. (1) Anterior abdominal wall; (2) GelPOINT device; (3) robotic arm one; (4) robotic arm three; (5) 30° “up” laparoscope; (6) uterine mass (From Gargiulo et al. 2012b)
Although robot-assisted single-incision laparoscopic myomectomy may be beneficial in patients with higher BMIs due to excellent cosmetic outcomes achieved with 4 cm skin incision (Gargiulo et al. 2012b), further studies are needed in order to definitively investigate the risk of umbilical hernia formation in this subset of patients based on its recently reported association with laparoscopic single-site approach (Gunderson et al. 2012).
Another useful adjunct to robotic myomectomy successfully implemented at our institution is flexible CO2 laser (Barton and Gargiulo 2012) (Fig. 12.8). The low thermal spread of laser energy, preserved seven degrees of freedom, sturdy design suitable for blunt dissection, as well as reliable hemostatic effect due to helium gas flow, make flexible CO2 laser a useful and practical tool in a variety of gynecologic applications, including robotic myomectomy. In particular, use of CO2 laser is expected to minimize the thermal damage of the myometrium, and therefore facilitate the healing process. Potential disadvantages of this approach include cost and fiber failure during extensive angling. At our institution, CO2 laser is considered based on surgeon preference in cases with leading tumor size <8 cm in diameter. In cases when the leading tumor diameter is >8 cm, the preference goes to the ultrasonic scalpel due to its better ability to control bleeding.
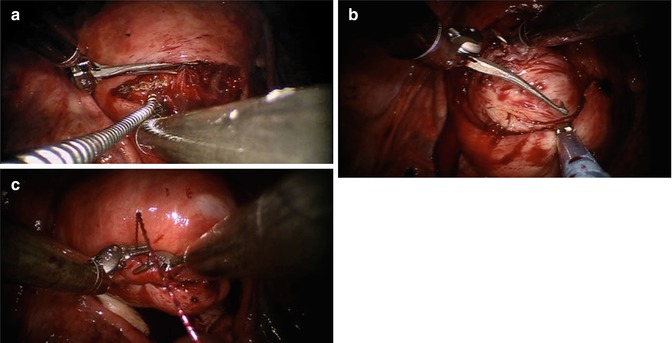

Fig. 12.8
CO2 Laser application during single incision robotic myomectomy. (a) Flexible laser fiber introduced through the assistant port is used for hysterotomy. (b) Tenaculum is used in conjunction with robotic shears and the assistant countertraction with Allis forceps. (c) After enucleation, the hysterotomy is closed in layers with large needle drivers (From Gargiulo et al. 2012b)
12.3 Conclusion
Despite evidence supporting the safety, feasibility, and improved outcomes following the minimally invasive approach to myomectomy, the majority of cases in the United States are performed by laparotomy (Liu et al. 2010). This may be due to the fact that majority of benign procedures are performed by community surgeons presented with unique challenges of mastering the minimally invasive techniques, including inconsistent training across generations and programs, dependency on various nonstandardized review courses to initiate learning, “on the job” training to attain competency, and perceived long learning curves needed to become proficient (Payne and Pitter 2011; Lenihan et al. 2008). Some of the challenges associated with adopting the laparoscopic technique include increased need for dexterity and hand–eye coordination in a two-dimensional field of view, long rigid instruments, tremor amplification, and nonergonomic body positioning of the surgeon (Stylopoulos and Rattner 2003). In addition to providing the benefits of three-dimensional view, tremor reduction and increased dexterity with seven degrees of freedom, the robotic approach acts as an enabling technology by facilitating successful completion of minimally invasive laparoscopic procedures with the same outcomes as more advanced surgeons (Lenihan et al. 2008). Lim et al. estimated the learning curve for robotic surgeries to be half the number of cases required to attain the same level of proficiency in laparoscopy (Lim et al. 2011). Other studies did not find significant difference in learning curve between the robotic and laparoscopic approach (Lenihan et al. 2008; Yacoub et al. 2010). Such variation in findings could be due to the differences in prior level of proficiency in laparoscopic and robotic surgery among the surgeons participating in the study. On average, 20–75 procedures are required to transcend the early learning curves associated with robot-assisted surgery (Geller et al. 2011).
Recent introduction of a dual console robotic system and greater recognition of the importance of minimally invasive training among residents and fellows at many programs across the country has led to interest in evaluating the outcomes of these approaches. Due to limitations in residency work duty hours, the amount of time spent on surgical training has also decreased and poses additional challenges to incorporating the novel minimally invasive approach into residency and fellowship training curriculum (Advincula and Wang 2009). In this context, the benefits provided by the dual console training are especially important since they provide an opportunity for the trainee to operate under direct supervision and at the same time as an attending physician (Smith et al. 2012). Potential obstacles for dual console training implementation include the possibility of litigation issues for the instructors and the need for designing protective measures (Lee et al. 2012).
With accessibility of the robotic approach in mind, it is important to remember that it is not intended to replace laparoscopy. Although safe and efficient, with outcomes at least on par with laparoscopy, robotic surgery is associated with significantly increased cost of procedure and prolonged operative times (Weinberg et al. 2011). The lack of tactile feedback is another sensible disadvantage in robotic myomectomy and other procedures depending on haptic feedback (Weinberg et al. 2011). Continued advancements and innovations in robotic sector are likely to eventually overcome the limitations currently posed by robotic approach and may lead to a paradigm shift in the field of gynecologic minimally invasive surgery.