Streptococcus pneumoniae
Haemophilus influenzae
Group F streptococcus
No organism isolated
3 (9%)
2 (6%)
1 (3%)
9 (26%)
8.1.3.3 Microbiology of late neonatal pneumonia
The predominant organisms isolated from the nasopharynx (nasopharyngeal aspirate) or trachea (endotracheal aspirate) of babies with VAP in both developing countries11 and Western settings8 are Gram-negative bacilli (Pseudomonas, Klebsiella, Acinetobacter, E. coli and other coliforms) and staphylococci (S. aureus and CoNS). GBS is only rarely isolated from babies with late pneumonia8 and thought to be a colonizing commensal, not a cause of late pneumonia. While routine endotracheal cultures predict poorly which babies will develop sepsis,12 they do provide data on colonizing organisms which might be causing pneumonia, so inform empiric antibiotic choice.
Late-onset GBS pneumonia is a potentially dangerous diagnosis to make because of the danger of missing another treatable cause of late pneumonia such as HSV or Chlamydia. If a baby 3 days of age or older develops respiratory distress and pneumonic changes, strong consideration should be given to a diagnosis of Chlamydia pneumonitis (Figure 8.1) or of HSV pneumonitis (Figure 8.2), because both need special tests for diagnosis. Untreated HSV pneumonitis often leads to fatal disseminated HSV infection (see Chapter 17).13
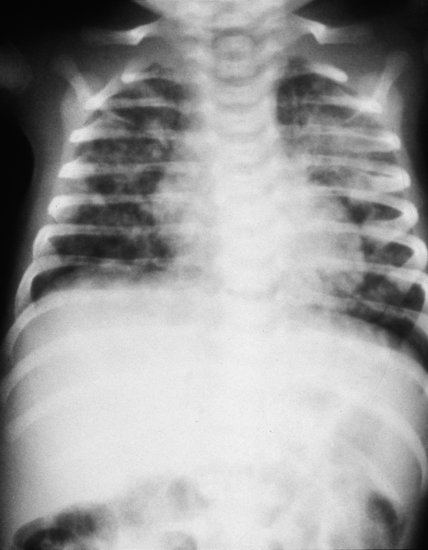
Figure 8.1 Chlamydia pneumonitis, age 14 days, showing perihilar changes and progressive clearing with treatment over 10 days with treatment.
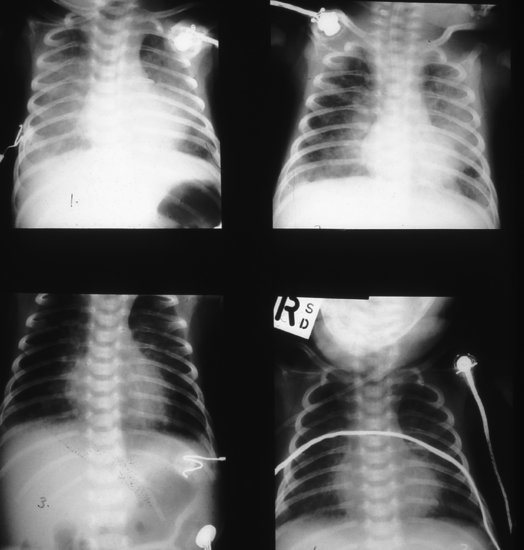
Figure 8.2 HSV pneumonitis: full-term infant with fast breathing aged 5 days; no history of maternal genital herpes; patchy pneumonitis; nasopharyngeal aspirate HSV-positive; infant recovered after acyclovir treatment.
In developing countries, there are few data on the organisms responsible for community-acquired pneumonia. In a Chinese study, 425 (56%) of 760 sputum samples from newborns with community-acquired pneumonia grew potential pathogens.14 Gram-negative organisms, mostly E. coli, Klebsiella pneumoniae and Haemophilus influenzae, were responsible for 65%; the rest grew mainly S. aureus or CoNS.14
8.1.4 Clinical features of pneumonia
The clinical features of neonatal congenital or early-onset pneumonia are indistinguishable from hyaline membrane disease. Signs of respiratory distress may include grunting, chest recession or retraction, nasal flaring, tachypnoea, dyspnoea, apnoea and cyanosis. Systemic signs may include lethargy, poor feeding and hypo- or hyperthermia. The signs of late pneumonia are similar but usually less dramatic.
Around 95% of infants with early-onset GBS infection with respiratory involvement develop clinical signs within 24 hours of birth (see Chapter 14). Respiratory distress starting between days 2 and 7 is very unlikely to be GBS pneumonia or hyaline membrane disease: the differential diagnosis includes HSV pneumonitis which must not be missed, virus infections (e.g. RSV, rhinovirus, influenza), Chlamydia pneumonitis, aspiration pneumonia, cardiac and other non-infectious causes.
8.1.5 Radiology of pneumonia
The radiologic appearance in early-onset GBS pneumonia may show a granular pattern, indistinguishable from hyaline membrane disease (Figure 8.3a) or more focal consolidation (Figure 8.3b).
Figure 8.3 (a) Group B streptococcal pneumonia: granular appearance resembling hyaline membrane disease. (b) Group B streptococcal pneumonia: hyperinflation and patchy right lower lobe changes.
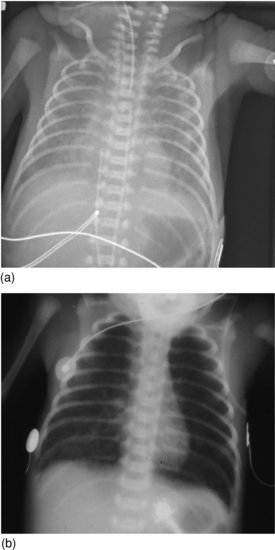
In a full-term infant, granular appearance (Figure 8.3a) is most likely to be due to bacterial pneumonia. In a pre-term infant, hyaline membrane disease is more likely, but bacterial pneumonia should be considered.
Ventilator-associated late-onset pneumonia can be difficult to distinguish from non-specific atelectasis. Persistent patchy consolidation is intuitively more likely to be due to bacterial infection, but there is no formal evidence.
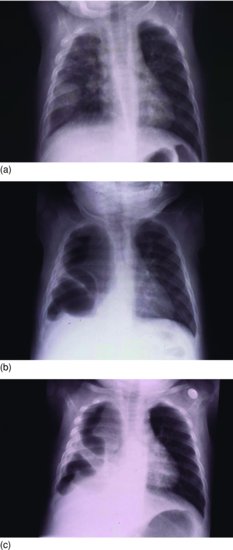
Staphylococcal pneumonia can cause focal consolidation which may progress to pleural effusion (Figure 8.4a), pneumatocoele formation (Figure 8.4b) and empyema (Figure 8.4c). While these radiologic features suggest S. aureus infection, other organisms including Gram-negative bacilli such as Klebsiella15 and E. coli16 can also cause pneumatocoeles, so the empiric antibiotics for an infant with pneumatocoeles should include Gram-negative cover.
Figure 8.4 (a) Staphylococcal pneumonia; round pneumonia and right pleural collection. (b) Staphylococcal pneumonia; large right pneumatocoele and pleural collection with mediastinal shift to left. (c) Staphylococcal pneumonia; loculated empyema.
Viral and Chlamydial infections are reported to cause patchy infiltrates and often hyperinflation, but no studies have compared radiologic appearance in neonatal viral and bacterial pneumonia. Studies in older children suggest the ‘classical’ radiologic features of viral and bacterial pneumonia overlap, while children can have simultaneous viral and bacterial pneumonia. For this reason it is unsafe to withhold antibiotics for a diagnosis of viral pneumonia made solely on radiologic grounds.
8.1.6 Antibiotic treatment of pneumonia
8.1.6.1 Antibiotic treatment of early-onset pneumonia
The choice of antibiotics for suspected early-onset pneumonia, which results from organisms acquired from the maternal birth canal, is the same as for early-onset sepsis (see Chapter 5) and depends on the likely pathogens based on local epidemiology.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


