Chapter 65 Respiratory Distress and Failure
The term respiratory distress is often used to indicate signs and symptoms of abnormal respiratory pattern. A child with nasal flaring, tachypnea, chest wall retractions, stridor, grunting, dyspnea, and wheezing is often judged as having respiratory distress. The magnitude of these findings is used to judge the clinical severity of respiratory distress. Although nasal flaring is a nonspecific sign, the other signs may be useful in localizing the site of pathology (Chapter 365). Respiratory failure is defined as inability of the lungs to provide sufficient oxygen (hypoxic respiratory failure) or remove carbon dioxide (ventilatory failure) to meet metabolic demands. Whereas respiratory distress is a clinical impression, the diagnosis of respiratory failure indicates inadequacy of oxygenation or ventilation or both. Respiratory distress can occur in patients without respiratory disease, and respiratory failure can occur in patients without respiratory distress.
Respiratory Distress
Respiratory Disease Manifesting as Respiratory Distress
Clinical examination is important in localizing the site of pathology (Chapter 365). Extrathoracic airway obstruction occurs anywhere above the thoracic inlet. Inspiratory stridor, suprasternal, chest wall, and subcostal retractions, and prolongation of inspiration are hallmarks of extrathoracic airway obstruction. By comparison, features of intrathoracic airway obstruction are prolongation of expiration and expiratory wheezing. Typical manifestations of alveolar interstitial pathology are rapid, shallow respirations, chest wall retractions, and grunting. The site of pathology can be localized and the differential diagnosis established on the basis of the clinical signs and symptoms (Tables 65-1 and 65-2).
Table 65-2 EXAMPLES OF ANATOMIC SITES OF LESIONS CAUSING RESPIRATORY FAILURE
| LUNG | RESPIRATORY PUMP |
|---|---|
| CENTRAL AIRWAY OBSTRUCTION | THORACIC CAGE |
| PERIPHERAL AIRWAY OBSTRUCTION | BRAINSTEM |
| Alveolar-Interstitial Disease | Spinal Cord |
| NEUROMUSCULAR | |
ARDS, acute respiratory distress syndrome; CNS, central nervous system.
Respiratory Distress without Respiratory Disease
Although respiratory distress most commonly results from diseases of lungs, airways, and chest wall, pathology in other organ systems can manifest as “respiratory distress” and lead to misdiagnosis and inappropriate management (Table 65-3). Respiratory distress resulting from heart failure or diabetic ketoacidosis may be misdiagnosed as asthma and improperly treated with albuterol, resulting in worsened hemodynamic state or ketoacidosis.
Table 65-3 NONPULMONARY CAUSES OF RESPIRATORY DISTRESS
| EXAMPLE(S) | MECHANISM(S) | |
|---|---|---|
| Cardiovascular | ||
| Central nervous system | Stimulation of brainstem respiratory centers | |
| Metabolic | Stimulation of central and peripheral chemoreceptors | |
| Renal | Renal tubular acidosis | Stimulation of central and peripheral chemoreceptors |
| Hypertension | Left ventricular dysfunction → increased pulmonary blood/water content | |
| Sepsis |
Cardiovascular Disease Manifesting as Respiratory Distress
A child with cardiovascular pathology may present with respiratory distress caused by 2 mechanisms: (1) decreased lung compliance and (2) cardiogenic shock (Table 65-4). Diseases that result in an increased pulmonary arterial blood flow (e.g., left-to-right shunts) or increased pulmonary venous pressure (e.g., left ventricular dysfunction from hypertension or myocarditis, obstructed total anomalous pulmonary venous return) cause an increase in pulmonary capillary pressure and transudation of fluid into the pulmonary interstitium and alveoli. The increased pulmonary blood and water content leads to decreased lung compliance and results in rapid shallow respirations.
Table 65-4 CARDIOVASCULAR PATHOLOGY MANIFESTING AS RESPIRATORY DISTRESS
Neurologic Disease Manifesting as Respiratory Distress
CNS dysfunction can lead to alterations in respiratory patterns. Increased intracranial pressure (ICP) may manifest as respiratory distress. Early rise in ICP results in stimulation of respiratory centers, leading to increases in the rate (tachypnea) and depth (hyperpnea) of respiration. The resultant decrease in PaCO2 and elevation of cerebrospinal fluid pH lead to cerebral vasoconstriction and amelioration of intracranial hypertension. Cerebral hemispheric and midbrain lesions often result in hyperpnea as well as tachypnea. In such situations, blood gas measurements typically show respiratory alkalosis without hypoxemia. Pathology affecting the pons and medulla manifests as irregular breathing patterns such as apneustic breathing (prolonged inspiration with brief expiratory periods), Cheyne-Stokes breathing (alternate periods of rapid and slow breathing), and irregular, ineffective breathing or apnea. Level of consciousness is most often impaired when abnormal breathing pattern from a brainstem disorder is present. Along with respiratory changes, other manifestations of CNS dysfunction and increased ICP may be present, such as focal neurologic signs, pupillary changes, hypertension, and bradycardia (Chapter 63). Occasionally, severe CNS dysfunction can result in neurogenic pulmonary edema (NPE) and respiratory distress, which may be due to excessive sympathetic discharge resulting in increased pulmonary venous hydrostatic pressure as well as increased pulmonary capillary permeability. Central neurogenic hyperventilation is characteristically observed in CNS involvement by illnesses such as Reye syndrome and encephalitis. Bradycardia and apnea may be due to CNS-depressant medications, poisoning, prolonged hypoxia, trauma, or infection (see Table 65-2).
Respiratory Failure
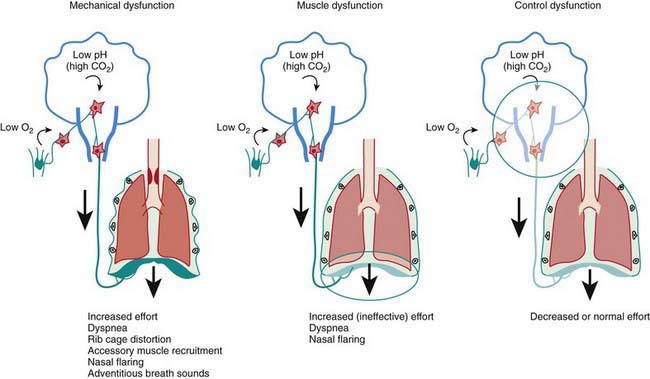
Respiratory failure occurs when oxygenation and ventilation are insufficient to meet the metabolic demands of the body. Respiratory failure may result from an abnormality in (1) lung and airways, (2) chest wall and muscles of respiration, or (3) central and peripheral chemoreceptors (Fig. 65-1). Clinical manifestations depend largely on the site of pathology. Although respiratory failure is traditionally defined as respiratory dysfunction resulting in PaO2 < 60 torr with breathing of room air and PaCO2 > 50 torr resulting in acidosis, the patient’s general state, respiratory effort, and potential for impending exhaustion are more important indicators than blood gas values.
Acute lung injury due to pneumonia, sepsis, aspiration, drowning, embolism, trauma, smoke inhalation, or drug overdose often leads to the acute respiratory distress syndrome (Table 65-5; Fig. 65-2).
Table 65-5 SIMPLIFIED CONSENSUS DEFINITION OF ACUTE LUNG INJURY
From Wheeler AP, Bernard GR: Acute lung injury and the acute respiratory distress syndrome: a clinical review, Lancet 369:1553–1564, 2007.
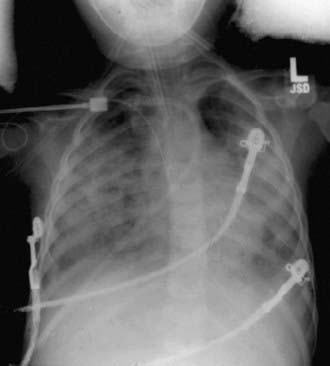
Figure 65-2 Frontal portal chest radiograph showing diffuse bilateral infiltrates consistent with acute lung injury.
(From Wheeler AP, Bernard GR: Acute lung injury and the acute respiratory distress syndrome: a clinical review, Lancet 369:1553–1564, 2007.)
Pathophysiology of Respiratory Failure
Ventilation-Perfusion Mismatch, Venous Admixture, Intrapulmonary Shunt
Normal VD/VT is around 0.33. VD/VT increases in states that result in decreased pulmonary perfusion, such as pulmonary hypertension, hypovolemia, and decreased cardiac output. Venous admixture and intrapulmonary shunting predominantly affect oxygenation, resulting in a PAO2-PaO2 (A-aO2) gradient without elevation in PaCO2. The reason is the greater ventilation of perfused areas, which is sufficient to normalize PaCO2 but not PaO2 because of their respective dissociation curves (Chapter 365). The relative straight-line relationship of hemoglobin-CO2 dissociation allows for averaging of PCO2 from hyperventilated and hypoventilated areas. Because the association between oxygen tension and hemoglobin saturation plateaus with increasing PaO2, the decreased hemoglobin-O2 saturation in poorly ventilated areas cannot be compensated for by well-ventilated areas where hemoglobin-O2 saturation has already reached near-maximum. This results in decreased SaO2 and PaO2. Elevation of PaCO2 in such situations is indicative of attendant alveolar hypoventilation. Examples of diseases leading to venous admixture include asthma and aspiration pneumonia, and those of intrapulmonary shunt include lobar pneumonia and acute respiratory distress syndrome.
Monitoring a Child in Respiratory Distress and Respiratory Failure
Clinical Examination
Clinical observation is the most important component of monitoring. The presence and magnitude of abnormal clinical findings, their progression with time, and their temporal relation to therapeutic interventions serve as guides to diagnosis and management (Chapter 365). The child with respiratory distress or failure should be observed in the position of greatest comfort and in the least threatening environment.
Pulse oximetry is the most commonly utilized technique to monitor oxygenation. Noninvasive and safe, it is the standard of care in bedside monitoring of children during transport, procedural sedation, surgery, and critical illness. It indirectly measures arterial hemoglobin-O2 saturation by differentiating oxyhemoglobin from deoxygenated hemoglobin using their respective light absorption at wavelengths of 660 nm (red) and 940 nm (infrared). A pulsatile circulation is required to enable detection of oxygenated blood entering the capillary bed. Percentage of oxyhemoglobin is reported as arterial oxyhemoglobin saturation (SaO2); however, the correct description is oxyhemoglobin saturation as measured by pulse oximetry (SpO2). This is because SpO2 may not reflect SaO2 in certain situations. It is important to be familiar with the hemoglobin-O2 dissociation curve (Chapter 365) in order to estimate PaO2 at a given oxyhemoglobin saturation. Because of the shape of the hemoglobin-O2 dissociation curve, changes in PaO2 above 70 torr are not readily identified by pulse oximetry. Also, at the same PaO2 level, there may be a significant change in SpO2 at a different blood pH value. In most situations, an SpO2 value greater than 95% is a reasonable goal, especially in emergency situations. There are exceptions, such as in patients with single ventricle cardiac lesions, in whom the pulmonary and systemic circulations are receiving blood flow from the same ventricle (e.g., after Norwood procedure for hypoplastic left heart syndrome), or with large left-to-right shunts (e.g., ventriculoseptal defect [VSD] and patent ductus arteriosus). In these types of pathophysiologic situations, a lower SpO2 is desired to avoid excessive blood flow to the lungs and pulmonary edema from the pulmonary vasodilatory effects of oxygen, and, in the patient with a single ventricle, diverting blood flow away from the systemic circulation. Because pulse oximetry recognizes all types of hemoglobin as either oxyhemoglobin or deoxygenated hemoglobin, it provides inaccurate information in the presence of carboxyhemoglobin and methemoglobin. Percentage of oxyhemoglobin is overestimated in carbon monoxide poisoning and methemoglobinemia. It should be recognized that dangerous levels of hypercarbia may exist in patients with ventilatory failure, who have satisfactory SpO2 if they are receiving supplemental oxygen. Pulse oximetry should not be the only monitoring method in patients with primary ventilatory failure, such as neuromuscular weakness and CNS depression. It is also unreliable in patients with poor perfusion and poor pulsatile flow to the extremities. Despite these limitations, pulse oximetry is a noninvasive, easily applicable, and effective means of evaluating the percentage of oxyhemoglobin in most patients.
Blood Gas Abnormalities in Respiratory Distress and Respiratory Failure
(See Chapters 52.7 and 365.)
Assessment of Oxygenation and Ventilation Deficits
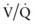 mismatch, intrapulmonary shunt, and diffusion defect, the status of alveolar hypoventilation could have a significant impact on PaO2/FIO2.
mismatch, intrapulmonary shunt, and diffusion defect, the status of alveolar hypoventilation could have a significant impact on PaO2/FIO2.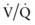 mismatch and alveolar capillary integrity.
mismatch and alveolar capillary integrity.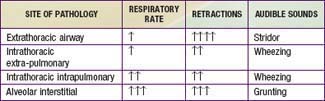
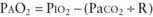
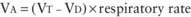
 ) is greater than the difference in ventilation (
) is greater than the difference in ventilation (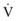 ). Perfusion in excess of ventilation results in incomplete “arterialization” of systemic venous (pulmonary arterial) blood and is referred to as venous admixture. Perfusion of unventilated areas is referred to as intrapulmonary shunting of systemic venous blood to systemic arterial circulation. Conversely, ventilation that is in excess of perfusion is “wasted”; that is, it does not contribute to gas exchange and is referred to as dead space ventilation. Dead space ventilation results in return of greater amounts of atmospheric gas (which has not participated in gas exchange and has negligible CO2) to the atmosphere during exhalation. The end result is a decrease in mixed expired P
). Perfusion in excess of ventilation results in incomplete “arterialization” of systemic venous (pulmonary arterial) blood and is referred to as venous admixture. Perfusion of unventilated areas is referred to as intrapulmonary shunting of systemic venous blood to systemic arterial circulation. Conversely, ventilation that is in excess of perfusion is “wasted”; that is, it does not contribute to gas exchange and is referred to as dead space ventilation. Dead space ventilation results in return of greater amounts of atmospheric gas (which has not participated in gas exchange and has negligible CO2) to the atmosphere during exhalation. The end result is a decrease in mixed expired P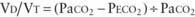
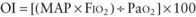
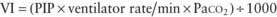
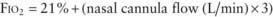
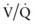 mismatch in patients with diseases that elevate pulmonary vascular resistance, such as persistent pulmonary hypertension of the newborn (PPHN), primary pulmonary hypertension, and secondary pulmonary hypertension due to chronic excess pulmonary blood flow (e.g., VSD) or collagen vascular diseases. NO is administered in doses ranging from 5 to 20 parts per million (ppm). Although administration of NO to unintubated patients is possible, it is usually administered to patients receiving mechanical ventilation through endotracheal tubes, because of the need for precision in NO dosing.
mismatch in patients with diseases that elevate pulmonary vascular resistance, such as persistent pulmonary hypertension of the newborn (PPHN), primary pulmonary hypertension, and secondary pulmonary hypertension due to chronic excess pulmonary blood flow (e.g., VSD) or collagen vascular diseases. NO is administered in doses ranging from 5 to 20 parts per million (ppm). Although administration of NO to unintubated patients is possible, it is usually administered to patients receiving mechanical ventilation through endotracheal tubes, because of the need for precision in NO dosing.


