Imaging modality
Advantages
Disadvantages
TVS
Easily available
Limited information on tubal or endometrial pathology
Relatively inexpensive
Can be limited in obese individuals
SIS
Assessment of endometrial pathology
Limited information on tubal pathology
HyCoSy
Visualization of ovaries, tubes, and endometrial lining
Patient discomfort
Limited in obese individuals
May require additional supply items
HSG
Better visualization of the entire fallopian tube
More invasive procedure
Possible increase in pregnancy rate
Radiation exposure
Less limited by body habitus
Risk of sensitivity to contrast
Not available in all facilities
MRI
Better method to assess leiomyomas or uterine anomalies
Expensive
Not limited by body habitus, less observer dependent, more reproducible
Not available in all facilities
Table 5.2
Reproductive imaging modality decision tree analysis

Clinical Case
A 34-year-old woman presents with a history of regular heavy menstrual bleeding. She is a gravida 0. She was tried on progestin medical therapy with no improvement. Pelvic ultrasound was normal. Endometrial biopsy showed hormonal effect with no other abnormality. A saline infusion sonography is ordered.
5.2 Pelvic Ultrasonography
Both transabdominal (TAS) and transvaginal sonography (TVS) are safe, noninvasive, and readily available in most office settings. Sonography can provide an easily accessible image of the uterus, ovaries, and other pelvic structures (pelvic kidney, appendix or adnexal masses).
5.2.1 Principles
Ultrasound images are obtained by emitting pulses of high-frequency sound and measuring the echoes that are reflected back to the transducer from interfaces between tissue structures of different impedance. The echoes are then transformed into real-time dynamic images of these structures. Most probes are curvilinear or convex, providing a wider field of view within a compact probe design. Most ultrasound images are obtained using B-mode (brightness) or gray-scale, displaying images in two dimensions. Ultrasound can also be used in the following modes: (1) M-mode to analyze cardiac motion, (2) color flow Doppler ultrasonography to measure the speed and direction of blood flow, or (3) three-dimensional (3D) ultrasonography, in which multiple B-mode images are combined into a 3D image that displays volume. Ultrasound frequencies in gynecology usually range between 3 and 7.5 MHz. Lower frequencies penetrate tissue more deeply with poorer resolution [1]. Conversely, higher frequencies penetrate tissue less deeply but give better resolution. Since ultrasound is a real-time technique, the performing provider can obtain additional information during the exam in regard to focal pain or lack of organ movement, which can be indicative of pelvic pathology.
5.2.2 Technical Considerations
Ultrasound can be performed via transabdominal or transvaginal approach, with the latter being preferred for gynecologic imaging since the probe is closer to the pelvic organs and allows better resolution of these structures. Large uterine or ovarian masses extending out of the pelvis, however, can be missed on transvaginal scanning; therefore, both approaches may be needed. The transabdominal approach is an option in a virginal patient and requires a full bladder to provide an acoustic window in order to fully delineate the pelvic structures. No prophylactic antibiotics or special analgesics are required for pelvic ultrasonography. A pelvic ultrasound is considered basically risk-free in non-pregnant patients. In pregnant patients, ultrasounds should only be performed if indicated [2]. However no studies have shown any abnormalities in children after prenatal ultrasounds [3].
Transvaginal probes should be disinfected after use and covered to prevent the transmission of infections between patients. Of note, the leakage rate when using condoms as vaginal probe covers was reported to be 0.9–2%, and as high as 8–81% when using commercial probe covers [4]. Agents available for high-level disinfection include glutaraldehyde, stabilized hydrogen peroxide (6%), orthophalaldehyde, peracetic acid, and peracetic acid-hydrogen peroxide [5], but compatibility of the probe and disinfectant should be confirmed per manufacturer’s instructions.
Pelvic ultrasound is best performed in the early follicular phase, when the endometrium is thin and endometrial pathology can be better visualized [6]. Heavy bleeding should not be present, because blood clots can be misinterpreted as polyps or adhesions [7]. A small amount of blood, however, can delineate the endometrial–myometrial interface (spontaneous sonohysterogram [8]).
Evaluation of the pelvis should proceed in the following systematic fashion, examining each area with respect to the following parameters:
- 1.
Uterus: Measurement of length, height, and width in longitudinal and transverse axes; size, number, and location of any leiomyomas , position and configuration of the uterus, thickness and appearance of endometrial lining, description of the junctional zone between endometrium and myometrium, any uterine malformations, or cervical abnormalities.
- 2.
Ovaries: Measurement of length, height, and width in longitudinal and transverse axes, number of antral follicles measuring between 2 and 9 mm, size and characteristics of any larger ovarian masses.
- 3.
Posterior cul de sac: Presence of any free fluid.
- 4.
Fallopian tubes: Normal fallopian tubes are rarely seen on pelvic ultrasound. A hypoechoic tubular or tortuous structure is suggestive of a hydrosalpinx [9], particularly if the “waist sign” is observed. The “waist sign” refers to diametrically opposed indentations in the wall of a cystic collection [10],
5.2.3 Limitations
Visualization of pelvic structures can be difficult when using a transabdominal approach on an obese individual. Overlying bowel can also interfere with visualization on both transabdominal and transvaginal ultrasound studies. There can be considerable variability in the quality and diagnostic capability of sonography dependent upon the experience and expertise of the ultrasonographer. Of note, it is often easier for a clinician to detect abnormalities while performing or observing a real-time dynamic scan rather than when reviewing previously acquired static images. Tubal patency cannot be assessed with standard ultrasonography. If endometrial pathology is suspected, saline infusion sonogram should be utilized.
5.2.4 Indications
Indications for pelvic ultrasonography include the following: (1) management of pelvic masses, (2) evaluation for ovarian torsion, (3) abnormal uterine bleeding, (4) uterine leiomyomas , (5) pelvic pain, (6) recurrent pregnancy loss, or (7) foreign bodies in the uterus. Ultrasonography is widely used for an infertility evaluation and includes (1) monitoring of follicle maturation (◘ Fig. 5.1), (2) assessment of endometrial thickness (◘ Fig. 5.2), (3) transvaginal oocyte aspiration, (4) ultrasound-guided embryo transfer [11], or (5) detection of hydrosalpinges (◘ Fig. 5.3a, b). Different publications have shown sensitivity of 86% [12] and specificity of 99.6% [13] for detecting a hydrosalpinx on transvaginal ultrasonography. 3D ultrasound can help to distinguish a hydrosalpinx from a complex ovarian cyst, since the entire tube is visualized spatially. Ultrasonography may be also helpful for the diagnosis of adenomyosis. Adenomyosis is suspected when the uterus is globular and bulky, with the myometrium being asymmetrically thickened. The junctional zone between endometrium and myometrium is usually ill-defined and heterogenous, which is caused by dilated endometrial glands in the myometrium.

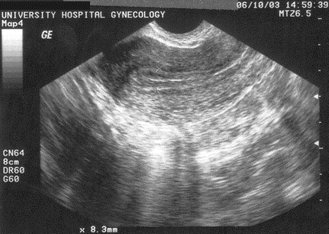


Fig. 5.1
Cystic structure representing a dominant ovarian follicle. Reproduced with permission from Lindheim SR, Uhler ML. In: Hurd WW, Falcone T, eds. Clinical reproductive medicine and surgery. St. Louis, MO: Mosby/Elsevier; 2007

Fig. 5.2
Trilaminar appearance of the endometrial echo. Reproduced with permission from Lindheim SR, Uhler ML. In: Hurd WW, Falcone T, eds. Clinical reproductive medicine and surgery. St. Louis, MO: Mosby/Elsevier; 2007

Fig. 5.3
a Hydrosalpinx. A hypoechoic tubular structure. Reproduced with permission from Lindheim SR, Uhler ML. In: Hurd WW, Falcone T, eds. Clinical reproductive medicine and surgery. St. Louis, MO: Mosby/Elsevier; 2007. b A hypoechoic tubular or tortuous structure is suggestive of a hydrosalpinx, particularly if the “waist sign” is observed. The “waist sign” refers to diametrically opposed indentations in the wall of a cystic collection
For the detection and classification of congenital uterine anomalies, a number of studies have demonstrated that 3D sonography is a reasonable alternative to a HSG or MRI procedure [14, 15]. 3D sonography allows one to visualize the external uterine contour as well as the internal morphology in the coronal plane . Bocca et al. [14] performed a prospective blinded study with 101 females who underwent routine HSG and 3D sonography as compared to surgical findings. The authors found 30 congenital anomalies (arcuate, unicornuate, bicornuate, septate uteri as well as uterine didelphys). Of the 30 congenital anomalies, all were correctly identified with 3D sonography, compared to only 10 correctly identified with HSG. Caliskan et al. [16] found that uterine anomalies visualized on 3D sonography are easier to interpret in the luteal phase compared to the follicular phase secondary to increased thickness and echogenicity of the endometrium. Ghi et al. [17] performed a prospective study on 284 nulliparous patients with at least three consecutive miscarriages who underwent 3D sonography. If the 3D sonography demonstrated a normal external and internal uterine contour, patients would undergo an office hysteroscopy. If the 3D sonography was abnormal, they underwent a concurrent hysteroscopy and laparoscopy. All 230 patients with negative 3D sonography results exhibited a normal uterine cavity at the time of office hysteroscopy. In the group with the abnormal 3D sonography, the presence of a Müllerian anomaly was correct in 52 out of 54 patients. 3D sonography in this study had a positive predictive value of 96.3% and a negative predictive value of 100%.
Currently only limited information is available to compare 3D sonography with MRI in the diagnosis of Müllerian anomalies. Bermejo et al. [15] found a concordance rate (kappa index = 0.880) between the two imaging modalities, however only 65 of the 286 females undergoing 3D ultrasound also had an MRI procedure.
The above studies suggest 3D sonography is an accurate and non-invasive tool to detect congenital uterine anomalies, with the advantages of less cost, lower morbidity, shorter examination time, and the availability to perform this procedure in the office setting.
5.3 Saline Infusion Sonography (SIS) or Sonohysterography (SHG)
5.3.1 Principles
Transvaginal ultrasonography alone has limited usefulness for demonstrating pathology in the endometrial cavity [18]. SIS enhances the ability to visualize lesions projecting into the uterine cavity. During the procedure, the endometrial cavity is filled with saline via a transcervical catheter, as first described by Nannini in 1981 [19]. Deichert et al. reported statistical equivalence among SIS, HSG, and hysteroscopy in regard to the evaluation of intrauterine pathology [20].
5.3.2 Technical Considerations
The SIS procedure should be performed between cycle days five to ten to avoid menstrual blood being misinterpreted as an intracavitary artifact. A follicular phase study ensures a thin endometrial lining, and avoids the possibility of an early pregnancy. Some providers recommend a urine pregnancy test prior to the SIS to decrease the risk of a concurrent pregnancy. If a patient is taking oral contraceptive pills, an SIS can be performed with less concern in regard to an occult conception and can facilitate timing of the procedure. Uterine cramping can occur, which usually responds well to the use of a nonsteroidal anti-inflammatory medication 30 min prior to performing the procedure.
Prior to the SIS procedure, informed consent should be obtained for the following possible sequelae: cramping, uterine bleeding, vasovagal reaction, or infection. Using an open-sided speculum, the cervix is cleansed with an antiseptic, and the SIS catheter is placed through the cervix. Different catheters can be used, including a standard size 5- or 7-Fr double-lumen intrauterine HSG catheter, a more rigid Goldstein sonohysterography catheter (Cook Ob/Gyn, Spencer, IN, USA), or a latex-free urethrane H/S Elliptosphere catheter (Akrad Laboratories, Cranford, NJ, USA), which contains an inflatable balloon (◘ Figs. 5.4 and 5.5). An 8-F pediatric Foley catheter can also be used, but it is more difficult to insert. Next, the speculum is removed and the transvaginal probe replaced. Sterile saline is then slowly injected into the uterine cavity, which leads to separation of the anterior and posterior uterine walls. Usually there is no need to insufflate the balloon. The uterus is then scanned in the longitudinal and transverse plane and 3D pictures can be obtained. If one encounters difficulty placing the catheter, a tenaculum can be placed on the cervix for straightening of the cervical canal, and a dilator utilized. If this is unsuccessful, the procedure can be repeated after having the patient take 400 μg of misoprostol orally 12 h prior to the SIS procedure for pre-procedure cervical dilation.



Fig. 5.4
Goldstein sonohysterography catheter (Cook Ob/Gyn, Spencer, IN, USA). Reproduced with permission from Lindheim SR, Uhler ML. In: Hurd WW, Falcone T, eds. Clinical reproductive medicine and surgery. St. Louis, MO: Mosby/Elsevier; 2007

Fig. 5.5
H/S Elliptosphere catheter with latex-free urethane (Cooper Surgical Inc., Turnbull, CT, USA). Reproduced with permission from Lindheim SR, Uhler ML. In: Hurd WW, Falcone T, eds. Clinical reproductive medicine and surgery. St. Louis, MO: Mosby/Elsevier; 2007
No uniform guidelines exist for antibiotic administration in patients undergoing SIS. For the HSG procedure, the American College of Obstetricians and Gynecologists (ACOG) recommends doxycycline 100 mg orally BID × 5 days if the patient has a history of pelvic inflammatory disease or if the procedure demonstrates dilated tubes [21]. There are no studies assessing the rate of post-SIS pelvic infections. One study reported four cases of pelvic infections after diagnostic or operative hysteroscopy that were successfully treated with antibiotics [22]. The decision to prophylactically treat a patient undergoing an SIS procedure is left to the provider, with some arguing to use the same criteria as outlined by ACOG for the HSG procedure.
5.3.3 Limitations
SIS should not be performed in a patient with documented intrauterine gestation, pelvic infection, or unexplained pelvic tenderness [23]. If a patient has a history of a known hydrosalpinx, some would defer the SIS procedure for the concern of a post-procedure infection. SIS can indirectly assess tubal patency by documentation of fluid in the posterior cul de sac; however, it cannot differentiate between laterality.
5.3.4 Indications
SIS can detect focal intrauterine lesions, such as polyps (◘ Fig. 5.6), submucosal uterine leiomyomas , or endometrial hyperplasia [24]. The incidence of polyps and submucosal leiomyomas in symptomatic premenopausal women is 33% and 21%, respectively [25]. The SIS procedure has been reported to be as accurate as hysteroscopy for the detection of focally growing lesions, with sensitivities for both procedures of approximately 96% [26]. Histologic tissue evaluation is recommended whenever intrauterine pathology is discovered, and blind endometrial biopsy may miss the pathology [27]. SIS in conjunction with 3D ultrasound can also help clarify any uterine malformations, such as septate versus bicornuate uterus, by depicting the outer uterine contour.


Fig. 5.6
Large endometrial polyp. Image provided by Steven Nakajima, MD, University of Louisville, Louisville, KY
Uterine leiomyomas are classified into three categories based on their location within the uterus, according to the European Society of Hysteroscopy Classification of Submucosal Fibroids [28]:
Class 1: Complete submucosal involvement with no myometrial involvement (intracavitary)
Class 2: Submucosal component involving less than half of the myometrium
Class 3: Submucosal involvement with an intramural component of greater than 50%
5.4 Hystero-Salpingo Contrastsonography (HyCoSy)
5.4.1 Principles
The HyCoSy can be the most comprehensive tool to evaluate the pelvic organs when compared to transvaginal ultrasound and SIS. In addition to gathering information about the uterus and the ovaries, the HyCoSy can also evaluate patency of the fallopian tubes, which are difficult to visualize on regular ultrasound if they are anatomically normal [29]. The HyCoSy uses contrast media that helps to visualize the structure of the fallopian tubes while transvaginal ultrasound is performed concomitantly. When compared to hysterosalpingogram (HSG), there is no exposure to radiation, and the procedure can be performed in the office rather than in the radiology suite.
5.4.2 Technical considerations
The HyCoSy procedure is normally conducted after performing an SIS procedure. The timing of the procedure, prerequisites, contraindications, and use of antibiotics are the same as for an SIS procedure. After evaluation of the uterine cavity, the intrauterine balloon is inflated with 3 mL of either fluid or air to occlude the lower uterine segment. For this reason, a Goldstein catheter cannot be used for the HyCoSy procedure. Next, a 20 mL syringe filled with both saline and air can be tilted intermittently to infuse 1–3 mL increments of saline followed by air [26]. Alternatively, a syringe with saline and air can be vigorously shaken immediately prior to infusion [30], or a commercial device is available that mixes the saline and air prior to the infusion. The mixture of saline and air can then be seen as “scintillations” travelling from the proximal interstitial portion of the tube to the distal fimbria and ovary [31] (◘ Figs. 5.7 and 5.8). If a tube fails to fill with the air/fluid mixture, it can be advantageous to ask the patient to roll slightly to position the tube superiorly. If no proximal or distal scintillations can be seen, this may represent true obstruction versus spasm in the cornual region. Once the study is completed, the balloon is deflated and all instruments removed from the vagina. The possibility of shoulder pain after the procedure secondary to peritoneal irritation by intraabdominal air should be discussed with the patient. This common side effect usually resolves after 24 h.
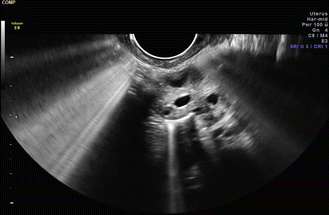


Fig. 5.7
Echogenic contrast identified in the proximal and distal portion of the right fallopian tube. Images provided by Steven Nakajima, MD, University of Louisville, Louisville, KY

Fig. 5.8
Echogenic contrast identified as scintillations flowing around the left ovary. Images provided by Steven Nakajima, MD, University of Louisville, Louisville, KY
Different contrast media have been used and evaluated, such as Hyskon (Pharmacia Laboratories, Piscataway, NJ, USA [32]) or Echovist-200 (Schering AG, Berlin, Germany), a galactose microparticle/air microbubble suspension that is not FDA-approved in the USA[33]. Fenzl [34] showed in a prospective randomized trial that using contrast media at body temperature caused less pain. To better characterize the fallopian tubes, Exacoustos and colleagues have combined 3D imaging with the HyCoSy procedure [35].
5.4.3 Limitations
Visualization of scintillations in the fallopian tubes may be more difficult in obese patients with a BMI greater than 35 kg/m2, as well as in case of distorted pelvic anatomy secondary to large uterine leiomyomas or adnexal masses [36]. Potential causes for false interpretation of HyCoSy findings include (1) missing distal occlusion when echogenic flow is seen in tube but not over the adjacent ovary, (2) presence of a tubal fistula, and (3) false tubal occlusion findings secondary to myometrial spasm at the cornua of the uterus [26].
5.4.4 Indications
Indications for a HyCoSy are usually fertility-related, since this procedure assesses tubal patency as well as evaluates the uterus. Multiple studies have compared the applicability of HyCoSy in regard to tubal patency with the HSG procedure and laparoscopy with chromopertubation. The concordance rate for these studies was between 72% and 86% [33, 37], with a 10% false occlusion rate and 7% false patency rate of the HyCoSy procedure when compared to laparoscopy. Some studies have evaluated pregnancy rates after laparoscopy, HSG, and HyCoSy and found no statistically significant difference [38, 39], thereby not supporting pregnancy enhancement after HyCoSy . Ayinda et al. [40] found no significant difference between HSG and HyCoSy in regard to frequency or severity of pain at different stages up to 28 days post procedure, and concluded that both tests are equally well tolerated, with HyCoSy avoiding pelvic radiation. Based on a retrospective chart review, Luciano et al. suggest HyCoSy as a method to accurately determine tubal occlusion after hysteroscopic sterilization [41].
5.5 Hysterosalpingogram (HSG)
5.5.1 Principles
The HSG procedure images the uterine cavity and fallopian tubes by injecting radiographic contrast media through the cervix and taking radiographs of the pelvis (◘ Fig. 5.9). The HSG has a high sensitivity but low specificity for the diagnosis of uterine cavity abnormalities and is therefore a good screening test [42]. One limitation of the HSG procedure is the differentiation between a septate and bicornuate uterus, since the external fundal contour is not imaged (◘ Fig. 5.10). This obstacle may be overcome by using the uterine push–pull technique to visualize the fundal contour of the uterus [43]. After tubal patency is verified, the uterus is mobilized by movement of the attached tenaculum, dispersing the contrast over the fundus of the uterus and thereby imaging the external fundal contour.




