FIGURE 53-1 The 50th, 75th, and 90th percentiles for maternal renal caliceal diameters measured using sonography in 1395 pregnant women from 4 to 42 weeks. (Redrawn from Faúndes, 1998.)
Evidence of functional renal hypertrophy becomes apparent very soon after conception. Glomeruli are larger, although cell numbers do not increase (Strevens, 2003). Pregnancy-induced intrarenal vasodilatation—both afferent and efferent resistance decreases—leads to increased effective renal plasma flow and glomerular filtration (Helal, 2012; Hussein, 2014). By 12 weeks’ gestation, the glomerular filtration rate is already increased 20 percent above nonpregnant values (Hladunewich, 2004). Ultimately, plasma flow and glomerular filtration increase by 40 and 65 percent, respectively. Consequently, serum concentrations of creatinine and urea decrease substantively across pregnancy, and values within a nonpregnant normal range may be abnormal for pregnancy (Appendix, p. 1289). Other alterations include those related to maintaining normal acid-base homeostasis, osmoregulation, and fluid and electrolyte retention.
 Assessment of Renal Function During Pregnancy
Assessment of Renal Function During Pregnancy
The urinalysis is essentially unchanged during pregnancy, except for occasional glucosuria. Although protein excretion normally is increased, it seldom reaches levels that are detected by usual screening methods. As discussed in Chapter 4 (p. 65), Higby and colleagues (1994) reported 24-hour protein excretion to be 115 mg with a 95-percent confidence level of 260 mg/day. There were no significant differences by trimester. Albumin constitutes only a small part of total protein excretion and ranges from 5 to 30 mg/day. From their review, Airoldi and Weinstein (2007) concluded that proteinuria must exceed 300 mg/day to be considered abnormal. Many consider 500 mg/day to be important with gestational hypertension. Quantification of urinary albumin-to-creatinine ratio (ACR) in a spot urine sample—ideally from a first-morning void—is helpful in estimating a 24-hour albumin excretion rate (AER), in which ACR (mg/g) approximates AER (mg/24 h). Some laboratories measure total proteins instead of albumin.
Stehman-Breen and associates (2002) found that 3 percent of 4589 nulliparas had idiopathic hematuria, defined as 1+ or greater blood on urine dipstick when screened before 20 weeks. They also reported that these women had a twofold risk of developing preeclampsia. In another study of 1000 women screened during pregnancy, Brown and coworkers (2005) reported a 15-percent incidence of dipstick hematuria. Most women had trace levels of hematuria, and the false-positive rate was 40 percent.
If the serum creatinine level in pregnancy persistently exceeds 0.9 mg/dL (75 μmol/L), then intrinsic renal disease should be suspected. In these cases, some determine the creatinine clearance as an estimate of the glomerular filtration rate. Sonography provides imaging of renal size, relative consistency, and elements of obstruction (see Fig. 53-1). Full-sequence intravenous pyelography is not done routinely, but injection of contrast media with one or two abdominal radiographs may be indicated by the clinical situation (Chap. 46, p. 931). The usual clinical indications for cystoscopy are followed. There is an approximate 5-percent complication rate of ureteroscopy done for stone removal during pregnancy (Johnson, 2012; Semins, 2009). Magnetic resonance (MR) imaging of renal masses has been shown to have excellent results (Putra, 2009).
Although renal biopsy is relatively safely performed during pregnancy, it usually is postponed unless results may change therapy. From a review of 243 biopsies in pregnant women, the incidence of complications was 7 percent—this compares with 1 percent in postpartum women (Piccoli, 2013). Lindheimer and colleagues (2007a) recommend its consideration for rapid deterioration of renal function with no obvious cause or for symptomatic nephrotic syndrome. We and others have found biopsy helpful in selected cases to direct management (Chen, 2001; Piccoli, 2013). Strevens and associates (2003) performed renal biopsy in 12 normal pregnant volunteers and reported that five had slight to moderate glomerular endotheliosis. In contrast, all 27 women with proteinuric hypertension had endotheliosis, and in all but one, it was moderate to severe.
 Pregnancy after Unilateral Nephrectomy
Pregnancy after Unilateral Nephrectomy
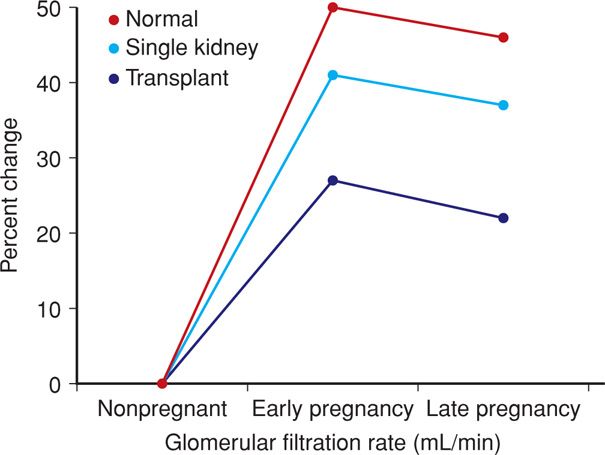
After removal of one kidney, and if the remaining kidney is normal, there is hypertrophy of renal function. In addition, with pregnancy, the surviving kidney undergoes further hypertrophy of function (Fig. 53-2). Because of this, women with one normal kidney most often have no difficulty in pregnancy (Baylis, 1991). Thorough functional evaluation of the remaining kidney is essential. Finally, there are no long-term adverse consequences of kidney donation done before pregnancy (Ibrahim, 2009).
FIGURE 53-2 Increased glomerular filtration rate in early pregnancy in normal women, those stable after unilateral nephrectomy, and those with a successful renal transplant. (Data from Newcastle-upon-Tyne, 1974–2006, courtesy of Dr. John Davison.)
URINARY TRACT INFECTIONS
These are the most common bacterial infections during pregnancy. Although asymptomatic bacteriuria is the most common, symptomatic infection includes cystitis, or it may involve the renal calyces, pelvis, and parenchyma—pyelonephritis.
Organisms that cause urinary infections are those from the normal perineal flora. Approximately 90 percent of Escherichia coli strains that cause nonobstructive pyelonephritis have adhesins such as P- and S-fimbriae. These are cell-surface protein structures that enhance bacterial adherence and thereby, virulence (Foxman, 2010; Hooton, 2012). These adhesins promote binding to vaginal and uroepithelial cells through expression of the PapG gene that encodes the P-fimbriae tip and by production of toxins and other virulence factors (Spurbeck, 2011).
Data suggest that pregnant women have more severe sequelae from urosepsis. The T-helper cell—Th1/Th2 ratio—reversal of normal pregnancy is discussed in Chapter 4 (p. 56). There are also various perturbations of cytokine expression that have been reported (Chaemsaithong, 2013). And maternal deaths have been attributed to E coli bearing Dr+ and P adhesins (Sledzińska, 2011). But even if pregnancy itself does not enhance these virulence factors, urinary stasis, vesicoureteral reflux, and diabetes predispose to symptomatic upper urinary infections (Czaja, 2009; Twickler, 1994).
In the puerperium, there are several risk factors that predispose a woman to urinary infections. Bladder sensitivity to intravesical fluid tension is often decreased as a consequence of labor trauma or conduction analgesia (Chap. 36, p. 676). Sensation of bladder distention can also be diminished by discomfort caused by an episiotomy, periurethral lacerations, or vaginal wall hematomas. Normal postpartum diuresis may worsen bladder overdistention, and catheterization to relieve retention commonly leads to urinary infection. Postpartum pyelonephritis is treated in the same manner as antepartum renal infections (McDonnold, 2012).
 Asymptomatic Bacteriuria
Asymptomatic Bacteriuria
This refers to persistent, actively multiplying bacteria within the urinary tract in asymptomatic women. Its prevalence in nonpregnant women is 5 to 6 percent and depends on parity, race, and socioeconomic status (Hooton, 2000). The highest incidence is in African-American multiparas with sickle-cell trait, and the lowest incidence is in affluent white women of low parity. Asymptomatic infection is also more common in diabetics (Schneeberger, 2014). Because in most women there is recurrent or persistent bacteriuria, it frequently is discovered during prenatal care. The incidence during pregnancy is similar to that in nonpregnant women and varies from 2 to 7 percent.
Bacteriuria is typically present at the first prenatal visit. An initial positive urine culture result prompts treatment, after which, fewer than 1 percent of women develop a urinary tract infection (Whalley, 1967). A clean-voided specimen containing more than 100,000 organisms/mL is diagnostic. It may be prudent to treat when lower concentrations are identified, because pyelonephritis develops in some women despite colony counts of only 20,000 to 50,000 organisms/mL (Lucas, 1993).
Significance
If asymptomatic bacteriuria is not treated, approximately 25 percent of infected women will develop symptomatic infection during pregnancy. Eradication of bacteriuria with antimicrobial agents prevents most of these. The American Academy of Pediatrics and the American College of Obstetricians and Gynecologists (2012), as well as a U.S. Preventive Services Task Force (2008), recommend screening for bacteriuria at the first prenatal visit (Chap. 9, p. 174). Standard urine cultures may not be cost effective when the prevalence is low. However, less expensive screening tests such as the leukocyte esterase-nitrite dipstick are when the prevalence is 2 percent or less (Rouse, 1995). Because of a high prevalence—5 to 8 percent—at Parkland Hospital, culture screening is done in most women. Susceptibility determination is not necessary because initial treatment is empirical (Hooton, 2012). Also, a dipstick culture technique has excellent positive- and negative-predictive values (Mignini, 2009). With this, a special agar-coated dipstick is first placed into urine and then also serves as the culture plate.
In some, but not all studies, covert bacteriuria has been associated with preterm or low-birthweight infants (Kass, 1962). It is even more controversial whether eradication of bacteriuria decreases these complications. Evaluating a cohort of 25,746 mother-infant pairs, Schieve and coworkers (1994) reported urinary tract infection to be associated with increased risks for low-birthweight infants, preterm delivery, pregnancy-associated hypertension, and anemia. These findings vary from those of Gilstrap and colleagues (1981b) and Whalley (1967). In most studies, asymptomatic infection is not evaluated separately from acute renal infection (Banhidy, 2007). A Cochrane database review by Vasquez and Abalos (2011) found that benefits of treatment for asymptomatic bacteria are limited to the reduction of the incidence of pyelonephritis.
Treatment
Bacteriuria responds to empirical treatment with any of several antimicrobial regimens listed in Table 53-1. Although selection can be based on in vitro susceptibilities, in our extensive experience, empirical oral treatment for 10 days with nitrofurantoin macrocrystals, 100 mg at bedtime, is usually effective. Lumbiganon and associates (2009) reported satisfactory results with a 7-day oral course of nitrofurantoin, 100 mg given twice daily. Single-dose antimicrobial therapy has also been used with success for bacteriuria. The important caveat is that, regardless of regimen given, the recurrence rate is approximately 30 percent. This may indicate covert upper tract infection and the need for longer therapy.
TABLE 53-1. Oral Antimicrobial Agents Used for Treatment of Pregnant Women with Asymptomatic Bacteriuria
Single-dose treatment
Amoxicillin, 3 g
Ampicillin, 2 g
Cephalosporin, 2 g
Nitrofurantoin, 200 mg
Trimethoprim-sulfamethoxazole, 320/1600 mg
3-day course
Amoxicillin, 500 mg three times daily
Ampicillin, 250 mg four times daily
Cephalosporin, 250 mg four times daily
Ciprofloxacin, 250 mg twice daily
Levofloxacin, 250 or 500 mg daily
Nitrofurantoin, 50 to 100 mg four times daily or 100 mg twice daily
Trimethoprim-sulfamethoxazole, 160/800 mg two times daily
Other
Nitrofurantoin, 100 mg four times daily for 10 days
Nitrofurantoin, 100 mg twice daily for 5 to 7 days
Nitrofurantoin, 100 mg at bedtime for 10 days
Treatment failures
Nitrofurantoin, 100 mg four times daily for 21 days
Suppression for bacterial persistence or recurrence
Nitrofurantoin, 100 mg at bedtime for pregnancy remainder
Periodic surveillance is necessary to prevent recurrent urinary infections (Schneeberger, 2012). For recurrent bacteriuria, we have had success with nitrofurantoin, 100 mg orally at bedtime for 21 days (Lucas, 1994). For women with persistent or frequent bacteriuria recurrences, suppressive therapy for the remainder of pregnancy can be given. We routinely use nitrofurantoin, 100 mg orally at bedtime. This drug may rarely cause an acute pulmonary reaction that dissipates on its withdrawal (Boggess, 1996).
 Cystitis and Urethritis
Cystitis and Urethritis
Lower urinary infection during pregnancy may develop without antecedent covert bacteriuria (Harris, 1981). Cystitis is characterized by dysuria, urgency, and frequency, but with few associated systemic findings. Pyuria and bacteriuria are usually found. Microscopic hematuria is common, and occasionally there is gross hematuria from hemorrhagic cystitis (Fakhoury, 1994). Although cystitis is usually uncomplicated, the upper urinary tract may become involved by ascending infection. Almost 40 percent of pregnant women with acute pyelonephritis have preceding symptoms of lower tract infection (Gilstrap, 1981a).
Women with cystitis respond readily to any of several regimens. Most of the three-day regimens listed in Table 53-1 are usually 90-percent effective (Fihn, 2003). Single-dose therapy is less effective, and if it is used, concomitant pyelonephritis must be confidently excluded.
Lower urinary tract symptoms with pyuria accompanied by a sterile urine culture may be from urethritis caused by Chlamydia trachomatis. Mucopurulent cervicitis usually coexists, and azithromycin therapy is effective. (Chap. 65, p. 1270)
 Acute Pyelonephritis
Acute Pyelonephritis
Renal infection is the most common serious medical complication of pregnancy. In a study of the 2006 Nationwide Inpatient Sample by Jolley and coworkers (2012), there were nearly 29,000 hospitalizations for acute pyelonephritis. Rates were highest for adolescents at 17.5 per 1000 and for Hispanic women at 10.1 per 1000. In another study of more than 70,000 pregnancies in a managed care organization, Gazmararian and colleagues (2002) reported that 3.5 percent of antepartum admissions were for urinary infections. The potential seriousness is underscored by the observations of Snyder and associates (2013) that pyelonephritis was the leading cause of septic shock during pregnancy. And in a 2-year audit of admissions to the Parkland Hospital Obstetrical Intensive Care Unit, 12 percent of antepartum admissions were for sepsis syndrome caused by pyelonephritis (Zeeman, 2003). There is also concern that urosepsis may be related to an increased incidence of cerebral palsy in preterm infants (Jacobsson, 2002). Fortunately, there appear to be no serious long-term maternal sequelae (Raz, 2003).
Clinical Findings
Renal infection develops more frequently in the second trimester, and nulliparity and young age are associated risk factors (Hill, 2005). Pyelonephritis is unilateral and right-sided in more than half of cases, and it is bilateral in a fourth. There is usually a rather abrupt onset with fever, shaking chills, and aching pain in one or both lumbar regions. Anorexia, nausea, and vomiting may worsen dehydration. Tenderness usually can be elicited by percussion in one or both costovertebral angles. The urinary sediment contains many leukocytes, frequently in clumps, and numerous bacteria. Bacteremia is demonstrated in 15 to 20 percent of these women. E coli is isolated from urine or blood in 70 to 80 percent of infections, Klebsiella pneumoniae in 3 to 5 percent, Enterobacter or Proteus species in 3 to 5 percent, and gram-positive organisms, including group B Streptococcus and S aureus, in up to 10 percent of cases (Hill, 2005; Wing, 2000). The differential diagnosis includes, among others, labor, chorioamnionitis, appendicitis, placental abruption, or infarcted leiomyoma. Evidence of the sepsis syndrome is common, and this is discussed in detail in Chapter 47 (p. 946).
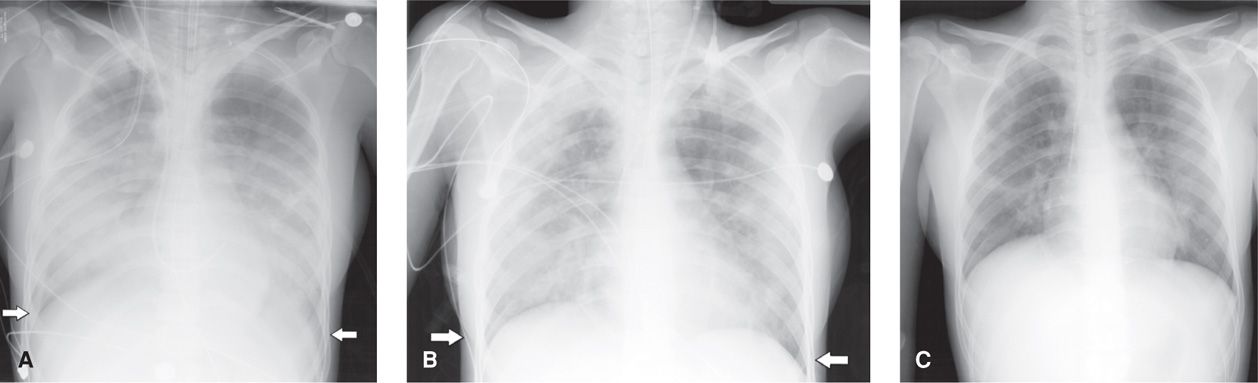
Plasma creatinine is monitored because early studies reported that 20 percent of pregnant women developed renal dysfunction. More recent findings, however, show this to be only 5 percent if aggressive fluid resuscitation is provided (Hill, 2005). Follow-up studies have demonstrated that this endotoxin-induced damage is reversible in the long term. Varying degrees of respiratory insufficiency from endotoxin-induced alveolar injury are manifest in up to 10 percent of women and may result in frank pulmonary edema (Cunningham, 1987; Sheffield, 2005; Snyder, 2013). In some cases, pulmonary injury may be so severe that it causes acute respiratory distress syndrome (ARDS) (Fig. 53-3).
FIGURE 53-3 A series of anterior-posterior projection chest radiographs of improving acute respiratory distress syndrome (ARDS) in a second-trimester pregnant woman with severe pyelonephritis. A. An extensive infiltrative process and complete obliteration of the diaphragm (white arrows) is seen. B. Improved aeration of lung fields bilaterally is noted as pleural disease resolves (arrows). C. Markedly improved visualization of the lungs fields with residual platelike atelectasis and normal appearance of the diaphragm.
Uterine activity from endotoxin is common and is related to fever severity (Graham, 1993). In the study by Millar and coworkers (2003), women with pyelonephritis averaged 5 contractions per hour at admission, and this decreased to 2 per hour within 6 hours of intravenous fluid and antimicrobial administration. As discussed in Chapter 47 (p. 942), ·-agonist therapy for tocolysis increases the likelihood of respiratory insufficiency from permeability edema because of the sodium- and fluid-retaining properties of those agents (Lamont, 2000). The incidence of pulmonary edema in women with pyelonephritis who were given ·-agonists was reported to be 8 percent—a fourfold increase over that expected (Towers, 1991).
Endotoxin-induced hemolysis is common, and approximately a third of patients with pyelonephritis develop anemia (Cox, 1991). With recovery, hemoglobin regeneration is normal because acute infection does not affect erythropoietin production (Cavenee, 1994).
Management
One scheme for management of acute pyelonephritis is shown in Table 53-2. Although we routinely obtain urine and blood cultures, prospective trials show them to be of limited clinical utility (Wing, 2000). Intravenous hydration to ensure adequate urinary output is the cornerstone of treatment. Antimicrobials are also begun promptly with the caveat that they may initially worsen endotoxemia from bacterial lysis. Ongoing surveillance for worsening of sepsis syndrome is monitored by serial determinations of urinary output, blood pressure, pulse, temperature, and oxygen saturation. High fever should be lowered with a cooling blanket or acetaminophen. This is especially important in early pregnancy because of possible teratogenic effects of hyperthermia (Chap. 14, p. 284).
TABLE 53-2. Management of the Pregnant Woman with Acute Pyelonephritis
Hospitalize patient
Obtain urine and blood cultures
Evaluate hemogram, serum creatinine, and electrolytes
Monitor vital signs frequently, including urinary output—consider indwelling catheter
Establish urinary output ≥ 50 mL/hr with intravenous crystalloid solution
Administer intravenous antimicrobial therapy (see text)
Obtain chest radiograph if there is dyspnea or tachypnea
Repeat hematology and chemistry studies in 48 hours
Change to oral antimicrobials when afebrile
Discharge when afebrile 24 hours, consider antimicrobial therapy for 7 to 10 days
Repeat urine culture 1 to 2 weeks after antimicrobial therapy completed
Antimicrobial therapy usually is empirical, and ampicillin plus gentamicin; cefazolin or ceftriaxone; or an extended-spectrum antibiotic were all 95-percent effective in randomized trials (Sanchez-Ramos, 1995; Wing, 1998, 2000). Fewer than half of E coli strains are sensitive to ampicillin in vitro, but cephalosporins and gentamicin generally have excellent activity. Serum creatinine is monitored if nephrotoxic drugs are given. Initial treatment at Parkland Hospital is ampicillin plus gentamicin. Some recommend suitable substitutes if bacterial studies show in vitro resistance. With any of the regimens discussed, response is usually prompt, and 95 percent of women are afebrile by 72 hours (Hill, 2005; Sheffield, 2005; Wing, 2000). After discharge, most recommend oral therapy for a total of 7 to 14 days (Hooton, 2012).
Persistent Infection. Generally, intravenous hydration and antimicrobial therapy are followed by stepwise defervescence of approximately 1°F per day. With persistent spiking fever or lack of clinical improvement by 48 to 72 hours, urinary tract obstruction or another complication or both are considered. Renal sonography is recommended to search for obstruction manifest by abnormal ureteral or pyelocaliceal dilatation (Seidman, 1998). Although most women with continuing infection have no evidence of obstruction, some are found to have calculi. Although renal sonography will detect hydronephrosis, stones are not always seen in pregnancy (Butler, 2000; Maikranz, 1987). If stones are strongly suspected despite a nondiagnostic sonographic examination, a plain abdominal radiograph will identify nearly 90 percent. Another option is the modified one-shot intravenous pyelogram—a single radiograph obtained 30 minutes after contrast injection—which usually provides adequate imaging (Butler, 2000).
In some women, MR imaging may disclose the cause of persistent infection (Spencer, 2004). Even without urinary obstruction, persistent infection can be due to an intrarenal or perinephric abscess or phlegmon (Cox, 1988; Rafi, 2012). Obstruction relief is important, and one method is cystoscopic placement of a double-J ureteral stent (Rodriguez, 1988). Because these stents are usually left in place until after delivery, they frequently become encrusted and require replacement. We have found that percutaneous nephrostomy is preferable because the stents are more easily replaced. Finally, surgical removal of stones may be required in some women (p. 1057).
Outpatient Management of Pyelonephritis. Outpatient management is an option for nonpregnant women with uncomplicated pyelonephritis (Hooton, 2012). Wing and associates (1999) have described outpatient management in 92 pregnant women who were first given in-hospital intramuscular ceftriaxone, two 1-g doses 24 hours apart. At this point, one third of the group was considered suitable for outpatient therapy, and these women were randomized either to discharge and oral antimicrobials or to continued hospitalization with intravenous therapy. A third of the outpatient management group was unable to adhere to the treatment regimen and was admitted. These findings suggest that outpatient management is applicable to very few pregnant women.
Surveillance
Recurrent infection—either covert or symptomatic—is common and develops in 30 to 40 percent of women following completion of treatment for pyelonephritis (Cunningham, 1973). Unless other measures are taken to ensure urine sterility, nitrofurantoin, 100 mg orally at bedtime given for the remainder of the pregnancy, reduces bacteriuria recurrence (Van Dorsten, 1987).
 Reflux Nephropathy
Reflux Nephropathy
Vesicoureteral reflux in early childhood can cause recurrent urinary tract infections, and thus, subsequent chronic interstitial nephritis was attributed to chronic pyelonephritis. Moreover, it was also found that high-pressure sterile reflux impaired normal renal growth. Combined, this leads to patchy interstitial scarring, tubular atrophy, and loss of nephron mass and is termed reflux nephropathy. In adults, long-term complications include hypertension, which may be severe if there is demonstrable renal damage (Diamond, 2012; Köhler, 2003).
Perhaps half of women with reflux nephropathy were treated during childhood for renal infections. Of these, many also had surgical correction of reflux as children, and these commonly have bacteriuria when pregnant (Mor, 2003). In the other half of women with reflux nephropathy, there is no clear history of recurrent cystitis, acute pyelonephritis, or obstructive disease (Diamond, 2012). Reports describing 939 pregnancies in 379 women with reflux nephropathy indicate that impaired renal function and bilateral renal scarring were associated with increased maternal complications (El-Khatib, 1994; Jungers, 1996; Köhler, 2003). Chronic renal disease and pregnancy outcome is discussed further on page 1061.
NEPHROLITHIASIS
Kidney stones develop in 7 percent of women during their lifetime with an average age of onset in the third decade (Asplin, 2012). Calcium salts make up approximately 80 percent of stones, and up to half of affected women have polygenic familial idiopathic hypercalciuria (Worcester, 2010). Hyperparathyroidism should be excluded. Although calcium oxalate stones in young nonpregnant women are most common, most stones in pregnancy—65 to 75 percent—are calcium phosphate or hydroxyapatite (Ross, 2008; Tan, 2013). Patients who have a stone typically form another stone every 2 to 3 years.
Contrary to past teachings, a low-calcium diet promotes stone formation. Prevention of recurrences with hydration and a diet low in sodium and protein is currently recommended (Asplin, 2012). Thiazide diuretics also diminish stone formation. In general, obstruction, infection, intractable pain, and heavy bleeding are indications for stone removal. Removal by a flexible basket via cystoscopy, although used less often than in the past, is still a reasonable consideration for pregnant women. In nonpregnant patients, stone destruction by lithotripsy is preferred to surgical therapy in most cases. There is limited information on the use of these procedures during pregnancy, and they are not generally recommended.
 Stone Disease During Pregnancy
Stone Disease During Pregnancy
The incidence of stone disease complicating pregnancy has been reported with widespread variability. At the low end, Butler and colleagues (2000) found the incidence to be 0.3 admissions per 1000 pregnancies in more than 186,000 deliveries at Parkland Hospital. In an Israeli population-based study, the incidence in nearly 220,000 pregnancies was 0.8 per 1000 (Rosenberg, 2011). In a population-based study from Washington state, Swartz and coworkers (2007) reported an incidence of 1.7 per 1000 pregnancies. Bladder stones are rare, but recurrent infection and labor obstructed by stones have been reported (Ait Benkaddour, 2006; Ruan, 2011).
Data are conflicting whether women with kidney stones have an increased risk for low-birthweight and preterm infants. The case-control study by Swartz and colleagues (2007) of 2239 women with nephrolithiasis reported excessive preterm delivery—10.6 versus 6.4 percent—compared with normal controls. The more recent nationwide population-based case-control study from Taiwan also reported 20- to 40-percent increases in low-birthweight and preterm births (Chung, 2013). To the contrary, a case-control study from Hungary reported that pregnancy outcomes, including preterm delivery, were similar in women with stones and normal controls (Banhidy, 2007). Comparable conclusions were drawn from the Israeli population-based study noted earlier (Rosenberg, 2011).
Diagnosis
There is some evidence that pregnant women may have fewer symptoms with stone passage because of urinary tract dilatation (Hendricks, 1991; Tan, 2013). That said, more than 90 percent of pregnant women with nephrolithiasis present with pain. Gross hematuria is less common than in nonpregnant women and was reported to be a presenting symptom in 23 percent of women described by Butler and associates (2000). In another study, however, Lewis and coworkers (2003) found that only 2 percent had hematuria. Sonography is usually selected to visualize stones, but as discussed above, many are not detected because hydronephrosis may obscure findings (McAleer, 2004). If there is abnormal dilatation without stone visualization, then the one-shot pyelogram may be useful. Transabdominal color Doppler sonography to detect presence or absence of ureteral “jets” of urine into the bladder has been used to exclude obstruction (Asrat, 1998).
Helical computed tomography (CT) scanning is the imaging method of choice for nonpregnant individuals, however, it is avoided during pregnancy if possible (Brown, 2010). If it is used, the slices can be tailored as needed (Chap. 46, p. 934). For pregnant women, White and colleagues (2007) recommend unenhanced helical CT and cite an average fetal radiation dose to be 7 mGy. These exigencies have led some to recommend MR imaging as the second-line test following nondiagnostic sonography (Masselli, 2013).
Management
Treatment depends on symptoms and gestational age (Semins, 2013). Intravenous hydration and analgesics are given. In half of women with symptomatic stones, infection will be identified, and this is treated vigorously. Although calculi infrequently cause symptomatic obstruction during pregnancy, persistent pyelonephritis should prompt a search for obstruction due to nephrolithiasis.
Approximately 65 to 80 percent of symptomatic women will have improvement with conservative therapy, and the stone usually passes spontaneously (Tan, 2013). Others require an invasive procedure such as ureteral stenting, ureteroscopy, percutaneous nephrostomy, transurethral laser lithotripsy, or basket extraction (Butler, 2000; Semins, 2010). The need for fluoroscopy limits the utility of percutaneous nephrolithotomy (Toth, 2005). In the case-control study cited above by Swartz and associates (2007), there were 623 procedures performed in 2239 symptomatic pregnant women, but less than 2 percent required surgical exploration.
As noted earlier, extracorporeal shock-wave lithotripsy is contraindicated in pregnancy. Watterson and coworkers (2002) described successful transurethral holmium:YAG laser lithotripsy in nine of 10 women. Semins and Matlaga (2010) found that ureteroscopic removal is also safe in pregnancy.
PREGNANCY AFTER RENAL TRANSPLANTATION
In 2013, there were approximately 97,000 registrants on the waiting list for renal transplantations through the Organ Procurement and Transplantation Network—OPTN (2013). The 1-year graft survival rate is 95 percent for grafts from living donors and 89 percent from deceased donors (Carpenter, 2008). Survival rates approximately doubled between 1988 and 1996, due in large part to the introduction of cyclosporine and muromonab-CD3 (OKT3 monoclonal antibody) to prevent and treat organ rejection. Since then, mycophenolate mofetil and tacrolimus have further reduced acute rejection episodes, however, the former is considered teratogenic (Briggs, 2011). In the report from the National Transplant Pregnancy Registry, 23 percent of fetuses exposed to mycophenolate had birth defects (Coscia, 2010). Importantly, resumption of renal function after transplantation promptly restores fertility in reproductive-aged women (Hladunewich, 2011; Lessan-Pezeshki, 2004). More than half of transplant recipients reported that they were not counseled regarding contraception (French, 2013).
 Pregnancy Outcomes
Pregnancy Outcomes
Coscia and coworkers (2010) reviewed the outcomes of 2000 pregnancies in transplant recipients as reported to the National Transplantation Pregnancy Registry. Most were treated with cyclosporine and tacrolimus, and approximately 75 percent of pregnancies resulted in a live birth. Similar outcomes were described for the Australian and New Zealand Transplant Registry by Wyld and associates (2013). Bramham and colleagues (2013) identified 105 pregnancies in renal transplant recipients and the United Kingdom Obstetric Surveillance System (UKOSS). Excluding nine abortions, there was only one perinatal death and 97 living children. Half were delivered before 37 weeks’ gestation, but only 9 percent before 32 weeks. Half were born weighing < 2500 g, and a fourth were growth restricted. Importantly, the incidence of fetal malformations was not increased, except in those who took mycophenolate mofetil (Coscia, 2010). Twin pregnancy has also been described following renal transplantation (Gizzo, 2014).
The incidence of preeclampsia is high in all transplant recipients (Brosens, 2013). In the UK National Cohort Study reported by Bramham and associates (2013), the incidence of preeclampsia was 22 percent. From their review, Josephson and McKay (2011) cite an incidence of a third of pregnancies but question the validity of this frequency. Importantly, in some cases, rejection is difficult to distinguish from preeclampsia. That said, the incidence of rejection episodes approximates only 2 percent (Bramham, 2013). Viral infections—especially polyomavirus hominis 1, also called BK virus, infections—are frequent. Also, gestational diabetes is found in approximately 5 percent. Both are likely related to immunosuppression therapy. Similar outcomes have been reported by several other investigators (Al Duraihimh, 2008; Cruz Lemini, 2007; Ghafari, 2008; Gutierrez, 2005).
Lindheimer (2007a) and Josephson (2011) and their coworkers recommend that women who have undergone transplantation satisfy several requisites before attempting pregnancy. First, women should be in good general health for at least 1 to 2 years after transplantation. Also, there should be stable renal function without severe renal insufficiency—serum creatinine < 2 mg/dL and preferably < 1.5 mg/dL—and < 500 mg/day proteinuria. Evidence for graft rejection should be absent for 6 months, and pyelocalyceal distention by urography should not be apparent. Moreover, hypertension should be absent or well controlled. And last, no teratogenic drugs are being given, and drug therapy is reduced to maintenance levels.
Cyclosporine or tacrolimus is given routinely to renal transplantation recipients (Jain, 2004). Cyclosporine blood levels decline during pregnancy, although this was not reported to be associated with rejection episodes (Thomas, 1997). Unfortunately, these agents are nephrotoxic and also may cause renal hypertension. In fact, they likely contribute substantively to chronic renal disease that develops in 10 to 20 percent of patients with nonrenal solid-organ transplantation (Goes, 2007).
Concern persists regarding the possible late effects in offspring subjected to immunosuppressive therapy in utero. These include malignancy, germ cell dysfunction, and malformations in the children of the offspring. In addition, cyclosporine is secreted in breast milk, and in at least one instance, it produced therapeutic serum levels in the nursing child (Moretti, 2003).
Finally, although pregnancy-induced renal hyperfiltration theoretically may impair long-term graft survival, Sturgiss and Davison (1995) found no evidence for this in a case-control study of 34 allograft recipients followed for a mean of 15 years.
 Management
Management
Close surveillance is necessary. Covert bacteriuria is treated, and if it is recurrent, suppressive treatment is given for the remainder of the pregnancy. Serial hepatic enzyme concentrations and blood counts are monitored for toxic effects of azathioprine and cyclosporine. Some recommend measurement of serum cyclosporine levels. Gestational diabetes is more common if corticosteroids are taken, and overt diabetes must be excluded with glucose tolerance testing done at approximately 26 weeks’ gestation. Surveillance for opportunistic infections from herpesvirus, cytomegalovirus, and toxoplasmosis is important because these infections are common. Some recommend surveillance for BK virus in women known to be infected (Josephson, 2011). Treatment is problematic.
Renal function is monitored, and as shown in Figure 53-2, the glomerular filtration rate usually increases 20 to 25 percent. If a significant rise in the serum creatinine level is detected, then its cause must be determined. Possibilities include acute rejection, cyclosporine toxicity, preeclampsia, infection, and urinary tract obstruction. Evidence of pyelonephritis or graft rejection should prompt admission for aggressive management. Imaging studies and kidney biopsy may be indicated. The woman is carefully monitored for development or worsening of underlying hypertension, and especially superimposed preeclampsia. Management of hypertension during pregnancy is the same as for patients without a transplant.
Because of increased incidences of fetal-growth restriction and preterm delivery, vigilant fetal surveillance is indicated (Chaps. 42, p. 842 and 44, p. 880). Although cesarean delivery is reserved for obstetrical indications, occasionally the transplanted kidney obstructs labor. In all women with a renal transplant, the cesarean delivery rate exceeds 60 percent (Bramham, 2013; Rocha, 2013).
POLYCYSTIC KIDNEY DISEASE
This usually autosomally dominant systemic disease primarily affects the kidneys. Its basic pathophysiology is one of a ciliopathy (Hildebrandt, 2011). The disease is found in 1 in 800 live births and causes approximately 5 to 10 percent of end-stage renal disease in the United States (Bargman, 2012). Although genetically heterogeneous, almost 85 percent of cases are due to PKD1 gene mutations on chromosome 16, and the other 15 percent to PKD2 mutations on chromosome 4 (Salant, 2012). Prenatal diagnosis is available if the mutation has been identified in a family member or if linkage has been established in the family.
Renal complications are more common in men than in women, and symptoms usually appear in the third or fourth decade. Flank pain, hematuria, proteinuria, abdominal masses, and associated calculi and infection are common findings. Hypertension develops in 75 percent, and progression to renal failure is a major problem. Superimposed acute renal failure may also develop from infection or obstruction from ureteral angulation by cyst displacement.
Other organs are commonly involved. Hepatic involvement is more common and more aggressive in women than in men (Chapman, 2003). Asymptomatic hepatic cysts coexist in a third of patients with polycystic kidneys. Approximately 10 percent of patients with polycystic kidney disease die from rupture of an associated intracranial berry aneurysm. Up to a fourth of patients have cardiac valvular lesions, with mitral valve prolapse and mitral, aortic, and tricuspid valvular incompetence.
 Pregnancy Outcomes
Pregnancy Outcomes
The prognosis for pregnancy in women with polycystic kidney disease depends on the degree of associated hypertension and renal insufficiency. Urinary tract infections are common. Chapman and coworkers (1994) compared pregnancy outcomes in 235 affected women who had 605 pregnancies with those of 108 unaffected family members who had 244 pregnancies. Composite perinatal complication rates were similar—33 versus 26 percent—but hypertension, including preeclampsia, was more common in women with polycystic kidneys. Pregnancy does not seem to accelerate the natural disease course (Lindheimer, 2007b).
GLOMERULAR DISEASES
The glomerulus and its capillaries are subject to numerous and various conditions and agents that can lead to acute and chronic diseases. Glomerular damage can be caused by several agents such as toxins or infections or from systemic disorders such as hypertension or diabetes. It may also be idiopathic. When there is capillary inflammation, the process is termed glomerulonephritis, and in many of these cases, an autoimmune process is involved. Glomerular disease or glomerulonephritis may result from a single stimulus such as that following group A streptococcal infections. However, it may be a manifestation of a multisystem disease such as systemic lupus erythematosus or diabetes (Sethi, 2012).
Persistent glomerulonephritis eventually leads to renal functional decline. Progression is variable and often does not become apparent until chronic renal insufficiency is diagnosed as discussed on page 1060. Lewis and Neilsen (2012) group glomerular injuries into six syndromes based on clinical patterns (Table 53-3). Some underlying disorders—examples include infections, vasculitides, and diabetes—can result in one clinical pattern in different individuals. Finally, within each of these categories, there are disorders encountered in young women, and thus, these may antedate or first manifest during pregnancy.
TABLE 53-3. Patterns of Clinical Glomerulonephritis
Acute Nephritic Syndromes: poststreptococcal, infective endocarditis, SLE, antiglomerular basement membrane disease, IgA nephropathy, ANCA vasculitis, Henoch-Schönlein purpura, cryoglobulinemia, membranoproliferative and mesangioproliferative glomerulonephritis
Pulmonary-Renal Syndromes: Goodpasture, ANCA vasculitis, Henoch-Schönlein purpura, cryoglobulinemia
Nephrotic Syndromes: minimal change disease, focal segmental glomerulosclerosis, membranous glomerulonephritis, diabetes, amyloidosis, others
Basement Membrane Syndromes: anti-GBM disease, others
Glomerular Vascular Syndromes: atherosclerosis, chronic hypertension, sickle-cell disease, thrombotic microangiopathies, antiphospholipid antibody syndrome, ANCA vasculitis, others
Infectious Disease-Associated Syndromes: poststreptococcal, infective endocarditis, HIV, HBV, HCV, syphilis, others
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree