Fig. 1.1
Trend lines demonstrate that as maternal age increases, live birth rate decreases, with a sharp decline at ~40 years. Ages are medians of ranges given in the literature
Some studies suggest that poorer prognosis for live birth after the age of 35 is directly linked to diminished ovarian reserve (DOR). In a study of patients with DOR undergoing IVF, Levi et al. [19] not only found higher rates of RPL in older patients (>40 compared to 35–40 and <35) but also found higher rates of recurrent miscarriage in patients with elevated FSH levels reflecting DOR in each of the age groups. They further reported that higher rates of aneuploidy were found in embryos of patients with RPL compared with those who did not have RPL [19]. This is consistent with other studies indicating that abnormal FSH and estradiol levels are associated with RPL and aneuploidy [20, 21]. Furthermore, infertility or subfertility could also play a role in the high rates of miscarriage at increased ages, but this will be discussed in more detail further on [22].
Aneuploidy is a significant cause of fetal loss and is directly linked to advanced maternal age (AMA ) . It accounts for approximately 50 % of first trimester abortions, 30 % of second trimester abortions, and 3 % of stillborn births [14]. Trisomy is the most common cause of aneuploidy by far, followed by other polysomies and structural anomalies inherited from parental anomalies.
The rate of aneuploidy in the products of conception from couples with RPL compared to the general population is debatable. Table 1.1 summarizes previous studies reports. Generally, we can conclude that fetal aneuploid y is positively associated with AMA [25, 26] and unexplained RPL [15], while it is negatively associated with the number of previous miscarriages [24, 27, 28].
Table 1.1
The rate of aneuploidy in RPL patients compared to the general population
Type of study | Number of cases | % aneuploidy in RPL | Rate of aneuploidy compared to the general population | Caveats | |
|---|---|---|---|---|---|
Carp et al. [20] | Retrospective analysis | 126 | 29 % | Lower | Only used in patients with three or more miscarriages |
Li et al. [16] | Retrospective, observational analysis | 105 | 32.4 % | Similar | |
Ogasawara et al. [24] | Retrospective analysis | 234 | 51.3 % | Similar | Abnormal karyotypes were less common as number of miscarriages increased |
Marquard et al. [25] | Retrospective cohort study | 50 | 78 % (>35 years old) | Higher | Patients with AMA were used |
Stephenson et al. [26] | Prospective cohort study | 197 | 40 % (>35 years old), 64 % (<35 years old) | Similar | |
Stern et al. [27] | Retrospective analysis | 94 | 57 % | Similar | Abnormal karyotypes were less common as number of miscarriages increased |
Sullivan et al. [28] | Retrospective analysis | 122 | 25.4 % | Lower |
Poor placentation and implantation failure are also associated with AMA. Higher rates of perinatal complications including preterm birth, stillbirth, and infertility as well as increased comorbidities including diabetes, obesity, and hypertension have been reported [29]. The increased complication rates of AMA could potentially lead to RPL in women who postpone their reproductive lives.
Karyotyping the Products of Conception
Karyotyping the products of conception (POC ) after the second miscarriage may provide reassurance to the couple as unknown etiologies tend to provoke more stress. Suigura-Ogasawara et al. [30] found that of the 70 % of couples with unexplained RPL, 41 % had abnormal karyotype in their subsequent pregnancy (see Fig. 11.1 in Chap. 11). This not only gives couples a reason for their miscarriage but it also increases the probability that their recurrent loss was due to chance alone, providing them reassurance to a live birth in their future pregnancy. Additionally, it may be more cost effective, with an average savings of $524 per couple, to analyze the POC before performing a standard work-up after two losses, especially in women >35 years old [31].
The Numbers Matter
The number of previous miscarriages is an important prognostic factor. Demonstrated in Fig. 1.2, as the number of miscarriages increases, the prognosis worsens [16–18, 30]. Li et al. [16] found that live birth rate was 64 % in couples with two miscarriages, but as low as 43.2 % in women with six or more miscarriages. Additionally, Lund et al. [18] found a 71.9 % success rate after 5 years in women with three previous losses versus 50.2 % success after six or more losses. Using a 5-year follow-up instead of risk per pregnancy is a beneficial estimate of success for patients because it approximates the overall outcome of having a child [18].


Fig. 1.2
Our figure shows that across studies, the live birth rate decreases as the number of previous miscarriages decreases
Maternal age must also be taken into account when developing a prognosis based on the number of previous miscarriages. The prospect for a live birth is dually affected as the quantity of miscarriages increases in couples with RPL because subsequent pregnancies occur at a later maternal age. Brigham et al. found that in 20-year-old women, the live birth rate after two miscarriages was 92 %, and 85 % after 5 miscarriages. These figures decreased to 77 % in 35-year-old women with two previous losses, and 62 % after five losses. At age 45, the numbers were much lower with a 60 % success rate after two losses, and 42 % after 5 losses [13].
Although the prognosis decreases as the number of miscarriages increases, it is important to reiterate that even women in their early forties with 5 or more losses still achieve a live birth rate of anywhere from 42 to 53 % [13]. Besides advising them about their worse prognosis, couples with five or more losses may warrant a different evaluation. As of now, all couples are evaluated in the RPL clinic equally, whether they are after two miscarriages or six. There is no literature that addresses this issue, but we suggest that further studies are needed for this subgroup to determine the most effective diagnostic evaluation and treatment available.
Two vs. Three
There are few implications in changing the definition of RPL from three or more to two or more losses. A small difference (30 vs. 33 %) in the index pregnancy of two as opposed to three pregnancy losses strongly supports the evaluation after two losses in order to provide the best outcome to couples [32].
Jaslow et al. [33] found no statistically significant differences in diagnostic factors identified in 1020 women with two losses versus three or more. Additionally, according to Bashiri et al. [4], there are no statistically significant differences in outcome for patients with primary RPL who had two versus three pregnancy losses. The study found there were higher levels of TSH in women with three versus two pregnancy losses (16.3 vs. 2.6 %, p = 0.033), and subsequent spontaneous pregnancy occurred more frequently in women with three pregnancy losses (91.7 vs. 77.4 %, p = 0.011). Low molecular weight heparin (LMWH) therapy was also used more in women with three or more losses (40.3 vs. 18.6 %, p = 0.016), and there were higher rates of chronic disease, unemployment, and consanguinity. Brigham et al. [13] also found no statistical differences between couples with 2 or more or 3 or more idiopathic miscarriages. Furthermore, Bhattacharya et al. [34] found that there was no statistical difference between two, three, and even four losses in estimating future pregnancy outcome in 143,595 pregnancies adjusted for maternal age, year of pregnancy, and smoking history.
The change in definition of RPL has led to earlier evaluation of couples with RPL in order to seek out an etiology. This shift raises two important considerations. First, some reviews evaluating the epidemiology of RPL struggle with the inclusion of patients with two losses because of the higher likelihood that the RPL is due to chance. As the number of miscarriages increases in a couple, the likelihood of the couple having an underlying cause increases and as a result, successful treatment reported in trials also increases. The second consideration involves a cost-benefit analysis, although this is beyond the scope of this review.
Primary vs. Secondary RPL
Primary RPL is defined as pregnancy loss with no previous live births, while secondary RPL refers to women with pregnancy loss and at least one live birth [35]. It has been suggested that secondary RPL couples make up 40–61 % of all people with RPL [35, 36]. Although some studies have found differences in couples with primary versus secondary RPL, the implications for the two groups remain to be seen.
Christiansen et al. [5] suggested that primary RPL may involve an innate immunological process after compiling data that found higher rates of thrombophilia, NK cell activity, and the effectiveness of allogenic lymphocyte immunization in primary RPL. Conversely, while secondary RPL may be linked more strongly to adaptive immunity, suggesting there are higher rates of antipaternal antibodies, HLA-DR3, and effectiveness of treatment with IVIg in secondary compared to primary RPL. The effectiveness of immunomodulating treatment will be discussed in a later chapter. However, these findings are currently more motivating for research opportunities.
Alternatively, Bashiri et al. [35] determined that there were no statistically significant prognostic differences in couples with primary versus secondary RPL in terms of live births (75.9 and 70.9 %, p = 0.262, respectively). However, higher pregnancy complications were observed in women with primary RPL such as preterm delivery, fetal growth restriction, and gestational diabetes mellitus after adjustment for age and gravidity. All diagnostic laboratory results were comparable in primary and secondary RPL patients, except for more cases of elevated prolactin in maternal blood. Most studies have agreed that there is no statistical difference [13, 16, 17, 37], and therefore, patients with primary and secondary RPL can be advised and evaluated in the same manner, although women with RPL should be monitored closely for obstetric complications.
While there is no difference in the evaluation of couples with primary versus secondary RPL, we often encounter unique circumstances in our clinic. In our area we have two special communities, the Ultra-Orthodox Jewish and the Bedouins. Both communities are characterized by families having 5–10 or more children. Couples are referred to the RPL clinic with secondary RPL after having 3, 4, and even 5 children. We desire to provide them with the counsel that they seek, but we struggle to prioritize these couples due to the limited time and resources in our publicly funded clinic, with a long queue of primary RPL couples. So far, our policy has been to perform a full patient history, discuss their prognosis, and advise them to continue to attempt to conceive. Then, if they insist on a further evaluation, we provide them the full evaluation. The logic behind this approach is that there is a possibility of acquiring pathology such as antiphospholipid syndrome, hypothyroidism, and uncontrolled diabetes.
Infertility and Superfertility
Infertility, defined as the inability to conceive after 1 year of regular intercourse without the use of contraceptives, has a negative impact on an RPL couple’s prospects of having a live birth [38]. Li et al. [16] found that those with RPL and a history of infertility had a lower live birth rate than those without (50.6 and 61.3 %). Additionally, women with infertility have reported a higher rate of fetal loss, with an odds ratio of 3.92 [38]. Studies supporting these results suggest that many infertile women are unknowingly having repeated early miscarriages [39]. Infertile women also continue attempting conception at an older age, which may contribute to their increased risk [22].
On the other hand, superfertility has also been associated with RPL. It is described as a monthly fecundity rate of >60 % (normal: ~20 %) [40], and the pathophysiology is attributed to dysregulation of the endometrium that allows implantation after the optimal window. Superfertile women conceive very easily but have complicated pregnancies and RPL [41] due to poorer placentation and more apparent pregnancy loss. Although it has not been described as a known etiology, superfertility is a new frontier of RPL that will be discussed later in this book.
Gestational Age
Gestational age is an important part of the couple’s history, as timing of previous losses could point to different etiologies. The majority of preclinical miscarriages are due to aneuploidy (70 %) [42] while thrombophilia and cervical incompetence are more causes of second trimester loss [43]. Still, no etiology is restricted to a certain gestational age, and every couple with RPL deserves a complete work-up.
Additionally, knowledge of the gestational age in the current pregnancy will provide reassurance as it advances. Detection of the fetal heartbeat confers the most reassurance for a live birth, since pregnancy loss occurs in only 2–6 % of women without RPL after fetal heartbeat detection [45]. This important finding should be visualized at 6 weeks gestation the latest [44]. It is thought that a couple with RPL has a three to five times higher chance of miscarriage after seeing a fetal heartbeat than the general population [14], as couples with RPL lose their pregnancy after a detected fetal heartbeat in 10.2–32 % of cases [45]. However, even if a fetal heartbeat is not determined around 6 weeks gestation, a repeat scan is indicated after 7 or more days before diagnosing an abortion [44].
RPL Etiology
Many entities have been examined to determine the source of RPL, but few have been significant enough to warrant investigation in all couples. Figure 1.3 demonstrates the proportions in which the etiologies contribute to RPL—parental chromosomal aberrations, uterine anomalies , endocrine abnormalities, autoimmune disorders, and thrombophilias. The next few sections discuss the known etiologies and the prognosis for patients who fall into these categories.
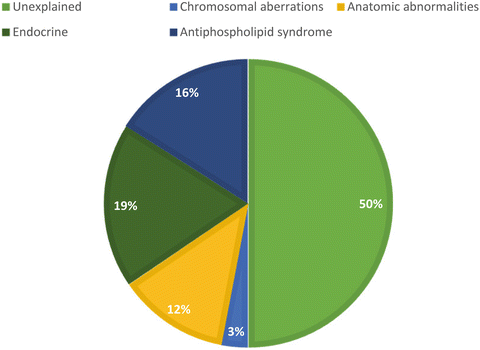

Fig. 1.3
Etiology of RPL
Parental Chromosomal Aberrations
Parental chromosomal anomalies account for 2–4 % of RPL in couples [32]. However, in unpublished data, Bashiri et al. have found a higher rate of chromosomal rearrangements at 11 % in an analysis of their patient database karyotyping approximately 500 couples. Translocations are the most common aberrations, followed by inversions, insertions, and mosaicism [32]. In light of its frequency and causality, the ASRM recommends chromosomal analysis of both partners during their initial evaluation [3]. Factors that have been associated with a higher likelihood of chromosomal aberrations include RPL in first-degree relatives and early age of onset of RPL [46]. The prognosis for couples with chromosomal abnormalities is difficult to express, with cumulative live birth rates ranging from 55 to 83 % for natural conception [3, 47, 48].
Couples with an abnormal karyotype can be advised to continue attempting pregnancy or they may be offered preimplantation genetic diagnosis (PGD). Continual attempts to conceive improve the chances of eventually having a child. Although the likelihood of miscarriage is higher for carriers, the prognosis for a live child is similar to those without aberrations [46].
Preimplantation genetic testing encompasses both screening and diagnostic measures and is currently the only intervention available to prevent pregnancy loss due to aneuploidy and chromosomal aberrations. PGD is a diagnostic tool for parents with known genetic anomalies. It has been found to reduce the rate of miscarriage in parents with structural chromosomal aberrations once they have become pregnant, but its ability to provide a better outcome for live birth compared to natural conception over time is controversial [49]. ESHRE determined that patients undergoing PGD for chromosomal abnormalities had the lowest pregnancy rate of all groups undergoing PGD, at less than 30 % per transfer. This was attributed to the concomitant infertility or subfertility in these patients [50] and the method used to detect healthy embryos. However, once pregnancy was achieved, women with chromosomal abnormalities had a live birth rate of 83 % in one clinical trial [51]. It is thought that a new method using microarray to determine healthy embryos will be more effective, but committees have not yet made recommendations [49]. Preimplantation genetic screening (PGS) is used largely for detecting aneuploidy and is reserved for patients with advanced maternal age , repeated implantation failure, and unexplained recurrent miscarriage. However, 11 studies have shown no benefit of PGS in terms of live birth. Moreover, PGS was suggested to negatively impact the pregnancy rate compared to natural conception in women with unexplained RPL [49, 50, 52]. Accordingly, a report by the American College of Obstetricians and Gynecologists (ACOG) concerning PGS does not support its use for AMA, recurrent unexplained miscarriage, or recurrent implantation failures [53].
Anatomic Abnormalities
Anatomic abnormalities cause 10–15 % of all RPL cases [32]. Septated uterus is the most common congenital abnormality, accounting for approximately 55–66 % of all uterine abnormalities in women with RPL [54]. Bicornuate and unicornuate make up the remaining 33 % of congenital anomalies. Acquired malformations that may contribute to RPL include polyps, fibroids, and intrauterine adhesions [54, 55]. The prevalence of uterine anomalies is approximately 3 times higher in those with RPL compared to the general population (12.6 and 4.3 %, respectively) [3]. Sugiura-Ogasawara et al. [56] found a live birth rate of untreated women with either a septated or bicornuate uterus of 59.5 %, with a cumulative birth rate of 78 %. These findings vary from others due to the fact that the septated and bicornuate uterus were combined in the study and usually treatment for a bicornuate uterus is not offered. For those with a septated uterus who wish to undergo treatment or continue to have pregnancy loss after diagnosis, hysteroscopic septectomy is offered although no randomized control trials have been performed to evaluate the effectiveness of treatment [57]. Nevertheless, Grimbizis et al. [58] performed a review of 9 retrospective and observational studies and found a cumulative live birth rate of 83.2 %, while another review of 18 retrospective trials have found an overall live birth rate of 45 % after hysteroscopic septectomy [59]. These two seemingly contradictory studies may represent the outcomes in different general groups of women. While Grimbizis et al. reviewed articles with some unspecified birth histories, Nouri et al. used articles that included women with complicated histories including infertility and RPL [58, 59]. Still, hysteroscopic septectomy should be discussed and offered to patients who have a history of RPL and a septate uterus due to its association with increased birth weight and decreased preterm delivery [60]. Unicornuate has the worst prognosis, with a live birth rate of 43.7 % with no available recommended surgical intervention [61].
Intrauterine adhesions (IUA) are also associated with RPL. Women with previous miscarriages have an increased risk of IUA, with a prevalence of 19–24 %, and an odds ratio of 1.99 in women with 2 or more miscarriages compared to one previous miscarriage [62, 63]. Although no previous studies have shown a poorer prognosis for women with RPL after repeated dilation and curettage, this may be a topic worth investigating due to its potential complications since a live birth rate of 71–88 % was found in women with IUA after treatment and a miscarriage rate of 26–30 % in untreated women [63, 64].
Lastly, it remains unclear if fibroids are associated with spontaneous miscarriage, although the size and location of the fibroids are important for prognosis determination [32]. Submucosal fibroids sized 5 cm or greater are more predictive of RPL and infertility, while intramural and subserosal remain to be associated with RPL [55, 65]. Saravelos et al. [66] found that women with RPL have a higher prevalence of fibroids than women with infertility. They suggest that women with repeated second trimester losses should be evaluated for fibroids and undergo myomectomy if present. It has been shown that myomectomy increases the chance of a successful pregnancy from 57 to 93 % [56], and should be considered in women with fibroids. See Chap. 7 for a further description of uterine anomalies.
Endocrine Abnormalities
Endocrine abnormalities affect both the implantation and maintenance of an embryo, and are the source of 17–20 % of all RPL [32]. Diabetes mellitus (DM) must be evaluated in patients with RPL using HbA1C, since uncontrolled DM increases the risk for fetal loss [3]. However, once controlled, DM is no longer a risk factor for RPL. Insulin resistance has been found at higher rates in women with RPL in the early stages of their pregnancy, with insulin resistance observed in 27 % of women with RPL compared to 9.5 % in the general population [67]. Zolghadri et al. [68] found that women with RPL had a higher prevalence of abnormal results in their glucose tolerance tests (17.6 % compared to 5.4 % of women without RPL). Furthermore, they found that those with abnormal glucose tolerance had better outcomes when taking metformin than those untreated, with an abortion rate of 15 and 55 % respectively. Therefore, these studies suggest insulin resistance is an important factor in RPL that warrants screening and treatment, although committee recommendations of this nature have not been made [67–69].
Overt hypothyroidism is seen in 0.2 % of pregnancies and subclinical hypothyroidism (SCH) is seen in 2–3 % [70]. All women with RPL should have their TSH levels monitored [3]. Although lowering the upper limit of a normal TSH level in pregnancy from 5.0 to 2.5 has been discussed, no conclusion in this matter has been made [71]. Pregnant women with known hypothyroidism must be monitored closely, as the rate of fetal loss is estimated to be 31 % in untreated women compared to 4 % in well-treated women [72]. Women with RPL have been found to have higher rates of hypothyroidism (10.5 %) and SCH (19 %) [73]. Furthermore, patients with SCH have been found to have higher rates of RPL [74]. A study by Benhadi et al. [75] of almost 2500 women showed that higher TSH levels were positively correlated with higher rates of pregnancy loss. Given the evidence that SCH may be associated with adverse outcomes, the Endocrine Society recommends that women with SCH are treated with T4 regardless of their thyroid antibody titers [76].
Thyroid autoimmunity has been directly linked with RPL [77], and 22–37 % [76] of women with RPL have positive thyroid antibodies (Tg-Ab or TPOAb), with as many as 10 % being thyroid antibody positive despite euthyroidism [74]. Kaprara et al. [78] reviewed 14 studies which found higher rates of miscarriage groups with thyroid antibodies compared to those without, with statistical significance in 10 out of 14. Additionally, 9 studies detected higher rates of auto-antibodies in groups with RPL compared to those without RPL, with statistical significance in 6 out of 9 [78]. Interestingly, high titers have not been shown to correlate with worse outcome than lower titers [70]. Levothyroxine is given for the treatment of autoimmune hypothyroidism [75]. Treatment for euthyroid women with positive thyroid antibody titers is not recommended [76], although levothyroxine has been shown in three studies to decrease fetal loss in thyroid antibody positive women [79–81].
The actual prevalence of PCOS in patients with RPL is not known, although Hudecova et al. [82] found similar live birth and miscarriage rates in both groups in a long-term follow-up. There is no correlation between PCOS and a high aneuploidy rate [82], and ultrasonographic polycystic ovaries and abnormal luteinizing hormone levels were not predictive of future miscarriage [84]. Although PCOS is not directly linked to RPL, hyperandrogenism, obesity, and hyperinsulinism are common sequelae in PCOS and have also been found in higher rates in women with RPL [81, 82].
Luteal phase defects have been studied for RPL and their association with endometrial dysfunction. However the lack of diagnostic criteria made the effects of varying luteal phase hormones on pregnancy impossible to quantify [83]. Therefore, according to the 2012 ASRM guidelines [3], progesterone treatment for luteal phase defects is not recommended. However, a recent systematic review by Carp made a compelling argument for treatment with dydrogesterone, a progestogen, in a review of 13 studies demonstrating a 13 % absolute reduction in the miscarriage rate among the treated vs. the untreated group [85].
Hyperprolactinemia is associated with RPL, and it may be involved in the pathogenesis of reproductive failure in patients with APS, PCOS, and hypothyroidism [3, 79, 86]. Hirahura et al. [86] found that treatment with bromocriptine was very effective for women with elevated prolactin levels, leading to a live birth rate of 85.7 %. On the other side, Li et al. [87] found that women with prolactin concentrations in the lower end of the physiological range had an increased risk of miscarriage when adjusted for all other factors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


