Quality-of-Life Issues in Gynecologic Oncology
DANA CHASE  LARI WENZEL
LARI WENZEL  RICHARD PENSON
RICHARD PENSON  JEANNE CARTER
JEANNE CARTER  DAVID CELLA
DAVID CELLA
INTRODUCTION
Health-related quality of life (HRQoL) is an increasingly important endpoint in clinical trials and a fundamentally important issue for patients. Quality of life (QoL) is a multifaceted and complex paradigm that reflects patients’ experiences with disease, treatment, and accompanying long-term sequelae (1, 2). While a specific definition of QoL may require articulating the patient’s status on physical, functional, emotional, and social well-being (3), it can also encompass the disparate aspects of patient demography (age, ethnicity, education, income), social circumstances (relationships and roles), and spiritual issues. It is no longer acceptable to pursue curative treatment with the hope of improving mortality without consideration of treatment morbidity and without including patient-centered decision-making and QoL implications. Therefore, this chapter provides an overview of state-of-the-science perspectives broadly incorporating medical interventions (e.g., recent clinical trials) that have influenced QoL and other patient-reported outcomes, and gynecologic cancer survivorship issues, including several challenging symptoms (e.g., fatigue, neurotoxicity, lymphedema) and long-term concerns (e.g., sexual dysfunction, reproductive concerns, emotional well-being).
QUALITY OF LIFE
QoL is an abstract, multidimensional construct that covers the subjective perceptions of the positive and negative aspects of patients’ experiences. We all know good QoL, but it is rarely simple. It integrates symptoms, physical, emotional, social, and cognitive functions, and reflects the impact of cancer and the side effects of treatment (4, 5). Figure 33.1 lists elements of QoL (6 – 8).
Considering well-being or QoL is as old as Aristotle’s concept of eudaimonia, or “good.” The first attempt to quantitatively measure the impact of cancer was described in 1949, when Karnofsky and Burchenal reported a simple 11 (0 to 10) point scale for the clinical evaluation of chemotherapy. This was simplified into the ECOG (Eastern Cooperative Oncology Group) Zubrod scale (0 = asymptomatic, 1 = symptomatic, 2 = functional for more than half the day, 3 = functional for less than half the day, 4 = moribund). Performance status probably remains the single most significant bias that contributes to the big differences between the results of phase 2 studies (9). Early QoL studies rapidly revealed considerable discrepancy between observers and between the doctor’s and his or her patient’s evaluation of the patient’s QoL, and it became clear that any method for measuring QoL, quintessentially subjective, would have to rely on patients themselves and not caregivers (10). Patient-reported outcomes (PROs) are now widely considered an excellent methodology to evaluate the utility of treatment. A simple composite measure of clinical benefit (measurements of pain [analgesic consumption and pain intensity], Karnofsky performance status, and weight) was used as the primary efficacy measure in a small but randomized study that led to Food and Drug Administration (FDA) approval of gemcitabine in advanced pancreatic cancer (11). Quality-adjusted time without symptoms of disease or toxicity of treatment (QTWIST) was an important addition as duration of symptoms, where they occur in the trajectory of the disease, and their source (disease or treatment) all influence their perception and impact (12). Large randomized controlled trials incorporating QoL endpoints have reported that docetaxel is associated with less neurotoxicity than paclitaxel (13), and erythropoietin (rHuEpo) significantly impacts anemia and fatigue (14). As physical functioning deteriorates, relational, spiritual, and psychologic issues become relatively more important (15, 16). QoL has been shown to predict survival in numerous disease settings (17, 18). In the Heart and Soul Study, a study of outcomes in 1,024 patients with coronary artery disease, depressive symptoms were more predictive of overall health than conventional measures of cardiac function such as ejection fraction and ischemia (19). Work conducted within the Gynecologic Oncology Group (GOG) has suggested that baseline QoL scores predict survival and may serve as an early barometer of patients who may or may not respond to aggressive treatment (20,21).
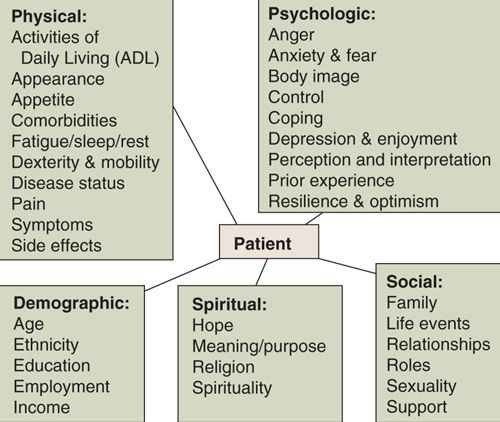
FIGURE 33.1. Elements of quality of life. Note: ADL, activities of daily living.
Source: Modified from Ferrell B, Smith SL, Cullinane CA, et al. Psychological well being and quality of life in ovarian cancer survivors. Cancer 2003;98:1061–1071; Gralla RJ. Silk purse in Atlanta: a commentary on SWOG 9509, an advanced non-small cell lung cancer trial. Oncologist 1999;4:188–190; Kornblith AB, Thaler HT, Wong G, et al. Quality of life of women with ovarian cancer. Gynecol Oncol 1995;59:231–242.
HRQoL is also recognized as a crucial component in medical decision-making research, for instance, if 2 treatments are shown to have equal efficacy, a HRQoL benefit could determine the preferable choice of therapy (22). Measurement and comparison of longitudinal changes in PROs are essential in evaluating clinical trials. Response-shift effects or the change in an individual’s internal beliefs, values, and perception based on health changes is viewed as a recognized confounder. Therefore, response shift effects should be assessed and adjusted for in clinical trials, allowing for a cautious approach to ensure that detected changes in HRQoL are truly due to treatment, intervention, or disease rather than measurement error (22).
Quantitative measure of patient outcomes is dependent on the use of validated instruments of psychometric data. Such questionnaires, or scales, list items (questions about particular constructs) in related domains (dimension or focus of behavior or experience). Retesting between subjects and over time establishes reliability (test-retest, interobserver variation, and correlation with related instruments), responsiveness, and applicability (generalizability or external validity). Good scales have to be (a) internally consistent (similar domains report agreement—Cronbach’s alpha ≥ 0.7), (b) stable (reliability coefficient), (c) equivalent or superior to other scales purporting to measure the same thing (kappa statistics), and (d) accepted by patients and experts. Approximately 500 instruments have been developed (23). Commonly used tools include the Functional Assessment of Cancer Therapy (FACT—with gynecologic and symptom subscales), the European Organization for the Research and Treatment of Cancer QLQ-C30 (EORTC QLQ-C30), Hospital and Anxiety Depression Scale (HADS), Profile of Mood States (POMS), Rotterdam Symptom Checklist (RSCL), MOS-Short Form 36 (SF-36), and Visual Analogue Scale (VAS).
The Functional Assessment of Cancer Therapy (FACT) instrument (scale) is a 27- (generic core) to 50-item compilation of specific subscales composed of physical, social/family, emotional, functional, and disease-specific well-being concerns (http://www.facit.org/) (24,25). The EORTC also developed a similar QoL instrument consisting of a core component applicable to all cancer patients and modules developed for specific cancer sites (26). EuroQol-5Dimension (EQ5D) utilizes a brief 5-item questionnaire covering mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, and a thermometer VAS ranging from 0 (worst imaginable health state) to 100 (best imaginable health state) (26).
For much of the last decade, the research agenda has been dominated by the comparison of instruments that evaluate the harder to measure aspects of clinical care (Fig. 33.2) (27,28). These studies have helped develop tools that have allowed important randomized clinical trials to now report QoL data (13,29). Formalizing the evaluation of HRQoL is becoming established, with ongoing debate over methodology and the distillation of a minimum set of criteria for assessing outcomes in cancer clinical trials that inform decisions in clinical practice (30). New modeling approaches that account for nonrandom omission of data are beginning to be accepted (31). Evaluating QoL helps describe populations, predict outcomes, and guide decisions (trade-offs and gambles), and can screen for dysfunction, help allocate resources, and improve awareness as patients approach end-of-life issues (32,33).
A novel approach to PROs is now funded by the National Institutes of Health (NIH). The Patient-Reported Outcomes Measurement Information System (PROMIS) network, which is part of the NIH Roadmap Initiative, aims to improve how PROs are selected and assessed in clinical research. PROMIS is establishing a publicly available resource of self-reported health domain measures, including those specifically targeted to cancer. More information can be found at http://www.nihpromis.org/ (24). Additional useful resources for QoL measurement include http://www.facit.org/, http://www.isoqol.org/, http://www.euroqol.org/, http://www.cancer.gov, and http://www.ql-recorder.com/.
FIGURE 33.2. Health-related quality of life—measuring the grey outcomes. Black and white outcomes: PFS, progression-free survival; OS, overall survival.
QOL OUTCOMES AND CLINICAL TRIAL UPDATES FOR ENDOMETRIAL, CERVICAL, OVARIAN, AND VULVAR CANCER
Integrated multidisciplinary care has appropriately become the necessary standard of the complex care of patients frequently in specialist centers, with goals of care changing over the course of the disease (Fig. 33.3).
Endometrial Cancer
Literature has identified quality-of-life data that may inform therapeutic choices for women with endometrial cancer. In an advanced endometrial cancer study, patients were randomized to whole-abdominal irradiation (WAI) versus doxorubicin-cisplatin (AP) chemotherapy. Although overall QoL did not differ between the 2 treatment groups, there were symptom-related differences (34). Specifically, WAI patients reported significantly higher fatigue than those who received AP chemotherapy for 7 cycles plus an additional cycle of cisplatin alone. Significant differences in functional alterations due to changes in bowel function were identified at the end of treatment (p < 0.01) and at 3 months (p < 0.01), with radiation associated with poorer scores. However, the AP group showed significantly higher peripheral neuropathy scores at the end of treatment and at 3 and 6 months posttreatment compared to the WAI group (p < 0.01). Therefore, although doxorubicin-cisplatin showed increased survival, the authors emphasized that the patients should be counseled regarding significant peripheral neuropathy that may have a significant impact on QoL.
FIGURE 33.3. Holistic care.
Notably, QoL has also been evaluated in comparisons of laparoscopy versus laparotomy (35,36). In both studies, QoL was superior in the laparoscopy group. In the GOG LAP2 trial, which compared differences in QoL between those endometrial cancer patients who underwent comprehensive surgical staging via laparoscopic technique versus traditional laparotomy, patients treated by laparoscopy had a superior QoL through 6 weeks postsurgery compared to those treated by laparotomy, likely due to it being a less invasive procedure, resulting in less pain, faster recovery, and a small but significant reduced length of hospital stay. However, except for a significantly better body image in the laparoscopy than the laparotomy group, these differences were not sustained at 6 months.
Response patterns for the LAP2 QoL data were reexamined by conceptualizing patient reports by content domains and development of constructs (i.e., sexual function, quality of relationships, body image, and fear of sex). No differences were found between the surgical groups, but the quality of the relationship, younger age, and being married increased the likelihood of responding to the items and better sexual function. Factors such as age, relationship quality, body image, and pain may place women with endometrial cancer at risk for sexual difficulties.
Radiation therapy is recommended for patients with high-risk features or advanced disease and is commonly utilized to prevent recurrence (37). The Postoperative Radiation Therapy in Endometrial Carcinoma (PORTEC-2) study examined the effects of high-dose intravaginal radiation therapy (HDIVRT) versus external beam radiation therapy (EBRT) (38). In the PORTEC-1 trial, it was observed that those women receiving EBRT versus observation had increased bowel and bladder symptoms in addition to QoL changes even 15 years out from treatment (39). With this information, the authors became skeptical of the actual benefit of EBRT in low to intermediate risk women. Then in PORTEC-2, the patients who received HDIVRT were found to have better QoL (38). Subsequently, HDIVRT has been gaining favor as a treatment modality, with supporting research demonstrating less morbidity (37,40–42) and excellent recurrence-free and overall survival rates (43) in comparison to EBRT (38,44). However, vaginal toxicities were noted with HDIVRT (dyspareunia, vaginal dryness, tightness, and shortening of the vagina), but further research is warranted to understand how these vaginal issues translate directly to sexual function for these women.
Finally, in the realm of endometrial cancer, obesity and its potential effect on QoL cannot be ignored. In fact, Fader et al. examined the association of obesity (BMI) and various QoL subscales (45). These authors highlighted the significant association of obesity with poor QoL in an otherwise highly curative cancer. Future research examining the role of diet and physical activity interventions is encouraged.
Cervical Cancer
In treatment of advanced cervical cancer, QoL considerations are primary. Several studies have demonstrated important QoL results that have influenced clinical trial paradigms. For example, a randomized phase 3 trial demonstrated that although there was greater toxicity in the cisplatin/paclitaxel (CP) regimen, there was no statistically significant difference in overall QoL scores between this treatment arm and the cisplatin (C) single-agent regimen. This finding, combined with the improved response rate and progression-free survival in the CP arm and a higher dropout rate in the C arm, suggests a worse outcome for the single-agent regimen. In this case, QoL measurement contributed to the conclusion that CP is superior to C alone with respect to response rate, progression-free interval, and sustained or improved QoL for patients treated with cisplatin versus cisplatin plus paclitaxel (20,46).
Several other trials have reported QoL as a secondary endpoint to use in addition to survival data when choosing chemotherapeutic regimens to treat advanced cervical cancer (47,48). These authors effectively argue that when determining if certain chemotherapy combinations may improve survival, it is critical to consider QoL, as toxicity may worsen. One such study prospectively assessed the impact of treatment with cisplatin alone (C), versus C in combination with topotecan (C+T) on QoL in patients with advanced or recurrent cervical cancer, and explored the prognostic value of baseline QoL scores. Results from this study, employing the FACT-Cx measure, indicated there were no statistically significant differences in QoL up to 9 months postrandomization, despite more hematologic toxicity in the C+T arm. The baseline FACT-Cx was also associated with predicting survival (48). This was the first advanced cervical cancer study to note that patient-reported QoL measures may be an important prognostic tool in advanced cervical cancer. Finally, in an analysis of 3 phase 3 trials, the physical well-being subscale proved to be independently prognostic of survival even when controlling for known contributing factors (49). These phase 3 trials have challenged a traditional study design for chemotherapeutic agents by incorporating PROs into the interpretations and implications of the relative value of these regimens. Clearly, QoL is a critical factor in palliative chemotherapy for recurrent and/or persistent advanced cervical cancer, where life expectancy is likely to be brief. Pelvic exenteration is one of the most radical, but potentially curative, treatment strategies for advanced or recurrent cervical cancer. The procedure is an en bloc resection of the pelvic organs (i.e., uterus, cervix, vagina, ovaries, lower urinary tract, and/or rectosigmoid colon). This procedure requires a motivated patient, with a good support network to assist in the recovery period (50). Provision of information and presurgical preparation for potential changes to a woman’s body (i.e., sexual function and ostomy care) are crucial for postoperative adjustment (50,51). Technological improvements in imaging have allowed for better selection of patients (no distant metastases) most likely to benefit from this extensive surgical procedure (52). The best candidates are those who are younger and have recurrent cervical cancer and pathologically negative surgical margins (52). This being said, the adjustment to life postexenteration is particularly challenging and requires intensive preoperative counseling.
Ovarian Cancer
Several phase 3 clinical trials have included longitudinal assessment of QoL as a secondary study endpoint. This information continues to influence a comprehensive interpretation of results, clinical trial design, and clinical decision making. For example, in a study of suboptimally debulked advanced ovarian cancer, although the addition of interval secondary cytoreduction to postoperative chemotherapy resulted in no notable long-term difference, a clinically significant QoL improvement was seen in both arms at 6 and 12 months after starting therapy. Fewer complaints of neurotoxicity at 6 months were reported among patients who did versus did not undergo interval secondary cytoreduction, likely due to the break in chemotherapy (53). In this study, it was also shown for the first time in ovarian cancer that baseline QoL scores could predict survival, attributed primarily to the lowest scoring quartile on the FACT-O. This has been demonstrated in other trials as well. Carey et al. described the Canadian experience of QoL and performance status as they relate to progression-free survival and overall survival in a frontline chemotherapy trial for ovarian cancer (54). Both global QoL and PS were associated with progression-free survival and overall survival, thereby confirming the findings of Wenzel et al. in the United States in 2005. QoL has also predicted survival for patients participating in a phase 3 trial comparing gemcitabine with pegylated liposomal doxorubicin (55).
A controversial randomized phase 3 trial in optimal stage III epithelial ovarian cancer showed that intravenous paclitaxel plus intraperitoneal cisplatin and paclitaxel significantly lengthened PFS and OS compared to intravenous paclitaxel and cisplatin (56). During active treatment, patients on the intraperitoneal arm experienced more HRQoL disruption, abdominal discomfort, and neurotoxicity than those receiving conventional intravenous therapy. However, only neurotoxicity remained significantly greater for intraperitoneal patients 12 months posttreatment, and generally for both groups QoL improved over time (57). Given the continued controversy regarding intraperitoneal therapy, QoL information will continue to provide clinically meaningful information from which to guide treatment decisions. Studies are currently being conducted to mitigate the added burden associated with intraperitoneal therapy while hopefully maintaining survival benefit.
Several additional recent phase 3 trials have incorporated QoL endpoints into advanced ovarian cancer clinical trials (58–63). For example, du Bois et al. compared paclitaxel plus cisplatin (PT) with paclitaxel plus carboplatin (TC) in patients with advanced ovarian cancer, evaluating QoL utilizing the EORTC QLQ-C30 questionnaire (59). There were significant QoL differences in favor of the TC arm, therefore leading to the conclusion that paclitaxel plus carboplatin is a more reasonable and better-tolerated treatment overall. In a subsequent study, Greimel et al. (62) examined hematologic and nonhematologic toxicity of the TC versus PT regimens and the effects of toxicity on QoL. Again, QoL was better in the TC regimen, resulting in confirmation that where 2 regimens have equal progression-free and overall survival, paclitaxel/carboplatin is preferable. A similar recommendation can be made from QoL data where it was demonstrated that gemcitabine plus carboplatin significantly improved PFS and response rate without worsening QoL (58). The relationship between cancer treatment efficacy, benefit, and QoL for elderly patients is also being studied. In one phase 3 trial comparing cisplatin and paclitaxel to carboplatin and paclitaxel, although QoL scores did not differ between elderly and younger patients, the elderly patients showed a higher rate of early treatment discontinuation. The authors speculated that this may be due to provider and/or patient unwillingness to continue treatment in the setting of toxicities, which might otherwise be managed differently in a younger population (64).
Finally, in 2010, Vergote et al. reported the results of a Gynecologic Cancer Intergroup Collaboration trial, which compared upfront debulking followed by chemotherapy to neoadjuvant chemotherapy (65). As the standard approach to treatment has historically been surgical cytoreduction followed by chemotherapy, this was the first randomized phase III trial of this alternative strategy. In this trial, patients completed QoL assessments using EORTC-validated instruments. The authors reported similar survival outcomes in the 2 groups, with both perioperative and postoperative morbidity being higher in the upfront surgery group. However, the QLQ-C30 global health scores were not significantly different between the 2 groups. The HRQoL measurement in this study did not seem to correlate with the “toxicity” (morbidity/mortality) demonstrated with upfront as compared to interval surgery. Future publications will hopefully elaborate on the QoL data to help explain this finding.
In the approach to recurrent ovarian cancer, tumor control without compromising HRQoL should be the goal of therapy (62). There have not been any phase III trials reported by the GOG that involve QoL assessments for recurrent ovarian cancer. There are, however, multiple international trials. The Gynecologic Cancer Intergroup used HRQoL measurements in their large trial of carboplatin and paclitaxel versus carboplatin and pegylated liposomal doxorubicin for recurrent platinum-sensitive ovarian cancer (CALYPSO) (66). QoL was measured every 3 months for 1 year from the date of enrollment. The doxorubicin arm was significantly less toxic. Patients treated with carboplatin and paclitaxel on this trial had longer-lasting and significant peripheral neuropathy, chemotherapy side effects in general, and worse body image. In addition, there were worse physical functioning and global QoL scores in these women at 3 months, although these were modest and did not persist at 6 months (67). This trial demonstrated the importance and utility of QoL data to challenge standard of care in large randomized phase III trials. Furthermore, in a German trial of nonplatinum doublets with topotecan versus topotecan alone, QoL measurements were also used (55). In this study, the doublet therapy neither provided survival advantage nor demonstrated differences in QoL scores. Combined therapy was associated in general with higher incidence of hematologic toxicity, yet this did not seem to be reflected in the QoL data. However, a detailed description of the data is lacking. In general, it appears that HRQoL data in the recurrent setting is deficient in terms of detailed descriptions of QoL disruptions and numbers of studies including QoL measurements.
Some may argue that QoL measurement in the recurrent setting is of particular importance being that therapy will often fail. This argument relates well to another sentinel paper published by Rustin et al. in 2010 (68). This study examined the impact of early versus delayed treatment of recurrent ovarian cancer based on CA-125 measurements exceeding twice the upper limit of normal. In patients in whom the CA-125 result was masked, treatment was initiated at clinical or symptomatic recurrence. The findings suggested that early treatment did not improve survival and, unfortunately, time to deterioration in QoL scores was shorter by several months in the early treatment group. This was seen across almost all QoL subscales. The QoL data here are noteworthy.
Unfortunately, effective screening programs and/or individual tests have yet to be clearly defined for ovarian cancer. Although QoL measurement in clinical trials evaluating therapeutic options for ovarian cancer is common, its use in the screening literature is limited. More commonly discussed is the impact of false-positive screening on QoL in cervical or breast cancer. Most QoL data in ovarian cancer screening lie in populations at high risk, such as those with genetic mutations undergoing risk-reducing salpingo-oophorectomy (RRSO). And, in the RRSO population, screening is more a process of early detection or diagnosis rather than a true screening test.
In general, the data are mixed regarding the negative impact of these screening tests on QoL (69). The U.S. National Cancer Institute’s Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial was a large trial to determine whether annual screening tests could reduce disease-related mortality (69). For the ovarian cancer screening group, women underwent annual CA-125 blood tests and pelvic ultrasounds. QoL was also measured using the SF-12 Physical and Mental Component Scales at each assessment. A cancer-specific distress scale was also used to measure satisfaction with the decision to participate in the trial as distress may indicate dissatisfaction and discomfort with the intervention of the trial. In this study, although not described as specific to ovarian cancer screening, participants with false-positive tests had higher levels of intrusive thoughts about cancer, yet this effect did not appear to be long-lasting. Women and participants with a first-degree relative with cancer were more likely to have these intrusive thoughts. The participants with false-negative tests were also less likely to comply with further testing. The authors concluded that in screening trials, it may be important to intervene on the part of the participants with false-negative results to decrease the potential stress involved in screening trials. Those assigned to the control group did not have statistically significant differences in HRQoL contrary to the belief that the mere ability to be involved in a cancer screening process alleviates stress associated with unknown risk for cancer.
There are several studies examining HRQoL in RRSO. In a cross-sectional study of 846 high-risk women, 44% had undergone RRSO instead of periodic gynecologic exams (70). Although “generic” QoL as measured by the SF-36 did not appear to differ between the groups, the RRSO group did report fewer concerns in general regarding their risk for cancer. Unfortunately, these women also had significantly more menopausal symptoms and worse sexual functioning relating to pleasure and discomfort than the women who underwent gynecologic exam alone. In 2009, Fang et al. described prospectively collected data on QoL, sexual functioning, body image, and depressive symptoms in 75 women undergoing either RRSO or serial screening (71). The women who underwent surgery reported poorer physical functioning, role limitations, pain, and social and sexual functioning at the 1-month assessment. Most deficits resolved by 6 months; however, the RRSO group had persistent menopausal complaints. Kahn et al also published on the impact of ovarian cancer screening on QoL. In this study of 135 patients, abnormal ovarian cancer screening results was associated with a significant decrease in the Mental Component Summary form of the Short Form-36 (72). Finally, the GOG also published initial data regarding RRSO and CA-125 in women at risk for ovarian cancer (GOG 199) (73). The study included QoL measurement; however, the data have not been published. Future work should perhaps target the RRSO group in terms of pre-operative counseling, postoperative recovery, and treatment of menopausal symptoms.
Vulvar Cancer
Vulvar cancers are rarer than other gynecologic cancers and predominantly occur in older women (74). Radical vulvar excisions are associated with poorer QoL and lower sexual function (75). Surgical treatment of vulvar cancer requires inguinal lymph node dissection (unilateral or bilateral) to assess regional metastasis; and wound breakdown and other postoperative surgical complications can be high in these cases (76–78). The GOG conducted a small prospective trial to evaluate reduction of lymphedema with standard closure versus closure with a fibrin sealant (Tisseel®, Baxter Health Care, Deerfield, IL) sprayed into the inguinal lymphadenectomy wound during surgical treatment for vulvar cancer. In this study, 150 women newly diagnosed with vulvar cancer were enrolled and randomized to either the standard closure or the fibrin sealant closure arm of the study, with 137 evaluable cases. The incidence of lower extremity lymphedema (LLE) was not statistically significant between the 2 groups; however, LLE rates were found to be elevated (60%–67%) at 6 months’ postoperatively in both the intervention and control groups (79). This study challenged the validity of lymphedema rates cited in previous research using retrospective study designs (36%–51% previously described in the literature) (80,81) and indicates that lymphedema rates may be greatly underestimated. Vulvar cancer set the groundwork for sentinel lymph node mapping, as lymphedema is a major issue among these patients. Nodal sampling seems to be a major contributory factor in lower extremity lymphedema (82).
CHALLENGES IN GYNECOLOGIC CANCER SURVIVORSHIP
Treatment side effects can influence short-term QoL for many women, and long-term QoL if sequelae persist or worsen over time. Recognition of and management for these issues can vary from simple strategies to improve QoL (i.e., managing nausea and vomiting) to complex psychosocial issues (e.g., reproductive concerns), for which clear guidelines may not exist. This section illustrates some of the current, potentially unique, and more complicated aspects of gynecologic cancer survivorship.
Pretreatment factors may influence a patient’s QoL both during and after treatment. Several authors have noted that QoL is affected by preoperative factors, such as education, lifestyle, general health, and obesity (83–85), all of which in turn may affect a patient’s ability to tolerate therapy. The interplay between cancer or cancer treatment symptoms and QoL is well documented in the gynecologic cancer literature (e.g., 86,87). Several prominent investigations deserve mention.
Cognitive issues—although there is a considerable and growing body of literature specific to cognitive impairment and fatigue during and after chemotherapy, only recently has this been considered within the gynecologic cancer cohort. For example, Hensley et al. were the first to study cognitive functioning prospectively in an ovarian cancer trial (88). Although they did not find significant decreases in cognitive functioning in women receiving paclitaxel, gemcitabine, and carboplatin in a phase II trial, they did note that highly educated women might suffer greater impairments, and thus, future trials should include cognitive functioning measures in their QoL assessments.
Fatigue—this occurs in 70% to 100% of patients with cancer, and is often underdiagnosed, inadequately treated, and hard to disentangle from other symptoms of physical compromise and psychologic distress (89). Fatigue, during and subsequent to cancer treatment, is a related, prevalent, and understudied issue for gynecologic cancer patients. Treatable causes of fatigue include anemia, malnutrition, medications, infections, pain, depression, insomnia, muscle dysfunction, and anticancer therapy (90,91). In an important QoL investigation, in 2,370 anemic cancer patients undergoing chemotherapy, recombinant human erythropoietin significantly improved patient-reported functional capacity and QoL independent of tumor response (14). Many authorities also advocate either energy conservation or pushing apparent boundaries with graded exercise and cognitive-behavioral therapy (92). Psychostimulants, such as short- and long-acting methylphenidate, have been initially positive, but more rigorous studies have suggested that there is questionable benefit over placebo or daily telephone calls from a research nurse (93).
Neurotoxicity—an increasingly documented long-term problem that cause sensory loss, pain, loss of function, loss of mobility, and for some women, loss of professional and recreational activity. Platinum compounds, the mainstay of treatment for most gynecologic malignancies (94), are associated with cumulative myelosuppression (particularly thrombocytopenia), neurotoxicity, and nephrotoxicity, as well as severe nausea and vomiting (95,96). Neurotoxicity, anemia, and nausea/vomiting all have well-known adverse effects on QoL. Paclitaxel in combination with a platinum compound is now considered the standard of care as first-line chemotherapy for advanced ovarian cancer (97,98). However, paclitaxel has a number of toxicities (e.g., granulocytopenia, neurotoxicity) that synergistically overlap those of the platins, and the coadministration of paclitaxel and a platinum compound can increase the frequency and/or severity of shared toxicities, especially neurotoxicity. Docetaxel is associated with significantly less neurotoxicity (99).
Administration of glutamine or the antidepressant venlafaxine may be helpful in cases of paclitaxel-induced neuropathy, and amifostine may provide protection from cisplatin-induced neuropathy (100,101). However, at present there appears to be no drug available to reliably prevent or treat chemotherapy-induced neuropathy (100,102). Therapeutic interventions for neurotoxicity remain controversial, with vitamins B6 and E possibly reducing the efficacy of alkylator chemotherapy. Nonpharmacologic approaches to treatment of chemotherapy-induced neuropathy are based on patient education regarding potential neuropathic side effects, the impact of these side effects on performance of daily activities, and related safety issues (e.g., tripping in the dark, driving).
It is worth noting that a new, reliable, and valid patient-reported measure is now available to document symptoms of neurotoxicity. The FACT/GOG-NTX measure consists of 11 items assessing sensory, motor, and hearing symptoms as well as possible functional impact. It was administered to 263 advanced endometrial cancer patients prior to each of 7 cycles of chemotherapy. Results of this study indicated that the patient-reported sensory symptom scores (sum of 4 item scores) increased significantly over the treatment duration (p < 0.001) in the paclitaxel/doxorubicin/cisplatin (TAP) arm compared to the doxorubicin/cisplatin (AP) arm. It was also noted that as few as 4 sensory symptoms in the FACT/GOG-NTX subscale can be used to reliably and sensitively assess chemotherapy-induced neurologic symptoms in clinical oncology without compromising the psychometric properties of the overall scale (103).
Lymphedema—can be challenging for gynecologic cancer patients, with the highest rates occurring in women treated for vulvar cancer (104–106). It is a chronic, progressive condition (104). The specific incidence of lymphedema of the lower extremity (LLE) following treatment for gynecologic cancer, as well as risk factors for LLE development, are not well documented in the literature, in contrast to research on upper extremity lymphedema (105). Findings from a retrospective study demonstrated that women treated with radical hysterectomy for cervical cancer were at an eightfold increased risk of developing LLE (106
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


