Changes Seen
Upper respiratory tract
• Hyperemia and edema of naso- and oropharynx resulting in rhinitis of pregnancy
Thorax/diaphragm
• Diaphragm rises by 4 cm
• Chest diameter increases by 2 cm
• Subcostal angle widens from 68° to 104°
Minute ventilation
• ↑↑↑(40–50 %)
Tidal volume
• ↑↑↑(40 %)
Respiratory rate
• ↔ to ↑(10 %)
O2 consumption
• ↑(20–30 %)
Lung Volumes (ml)
TLC
• ↔to ↓(5 %)
ERV
• ↓(15–20 %)
RV
• ↓ (20–25 %)
FRC
• ↓ (20–30 %)
VC
• ↔
IC
• ↑(5–10 %)
IRV
• ↔
Spirometry
FEV1 (L/min)
• ↔
FVC
• ↔
Diffusion capacity
• ↔
Arterial Blood Gases
PaO2 (mm Hg)
• 105–106 in first trimester and 101–106 by third trimester
PaCO2 (mmHg)
• 28–29 in first trimester and 26–30 by third trimester
pH
• 7.43
HCO3 (mEq/L)
• 17–18
Effect of Pregnancy on Asthma
The course of asthma during pregnancy is variable. The majority of patients who improve in pregnancy tend to worsen in the postpartum period and vice versa [1]. In general, asthma improves toward the end of the pregnancy, including labor and delivery. However, the rate of asthma exacerbations is increased between gestational weeks 17 and 32 [1, 2]. This may in part be due to medication noncompliance during the earlier part of the pregnancy upon discovery of the pregnancy but may also have to do with other pregnancy-related factors such as esophageal reflux, nasal congestion, hormonal factors, and alterations in immunity that may result in increased susceptibility to infections. The major predictor of disease course is the severity of asthma prior to the pregnancy, but race and obesity may also play a role. African American and Hispanic women are more likely to have asthma exacerbations. Poor compliance with medications and difficulties with access to medical services may be important confounders. Additionally, obese women tend to have more severe asthma as both asthma and obesity share a common inflammatory pathway at the cellular level. Asthma also tends to behave in a similar fashion in subsequent pregnancies.
Effect of Asthma on Pregnancy
While well-controlled asthma does not appear to have adverse consequences during pregnancy, poorly controlled asthma may negatively impact some maternal and fetal outcomes.
In the largest study performed to date on over 37,000 women with asthma and over 280,000 controls, asthmatic women were more likely to have pregnancies complicated by miscarriage, antepartum and postpartum hemorrhage, anemia, and depression [3]. However, the risk of other negative outcomes such as gestational hypertensive disorders and stillbirths was not significant in this study. In other large studies, a small, but statistically significant risk of perinatal mortality, preeclampsia, and preterm deliveries have been reported [4, 5]. A more recent retrospective cohort study performed in 12 clinical centers in the United States has shown increased risk of preeclampsia, gestational diabetes, and all preterm births [6]. Secondary analysis of a recent randomized controlled trial showed that women with perception of good asthma control had a reduced risk of planned cesarean deliveries, asthma exacerbations, and preterm birth [7]. In the same study, women with increased anxiety had a higher risk of exacerbations. There is some evidence suggesting that poorly controlled asthma also confers an increased risk of small for gestational age, and low birth weight [8]. Growth restriction may, however, be confounded by smoking. Babies born to severe asthmatics are possibly more likely to have congenital anomalies [5].
Management/Treatment
General Principles and Management
The treatment of asthma involves assessment and management from preconception to the postpartum period. Please refer to Table 11.3 and Figure 11.1 for a general overview of the classification and management of chronic asthma.
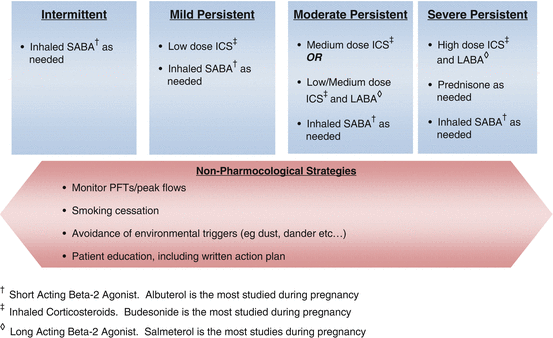

Fig. 11.1
Management of chronic asthma during pregnancy
There are four general components of asthma care, irrespective of gestational age. These are (1) monitoring of respiratory status, (2) avoidance of possible triggers, (3) patient education, and (4) pharmacological treatment. Patients should get a baseline spirometry and be instructed in how to follow their peak expiratory flow rate (PEFR) at home. Ideally, this should be done twice a day in patients with persistent disease. Since pregnancy does not affect flow rates, reductions in these numbers usually indicate a worsening degree of airflow obstruction and should prompt quick medical evaluation. Second, it is critical that patients avoid their known triggers to asthma including tobacco, dust, extreme temperatures, and allergens such as pollen and pet dander. Third, patients need to be educated about their disease. Pregnancy constitutes a perfect window to educate women given the multiple contacts with providers increased motivation due to concerns for fetal well-being. Trigger control from washing bed sheets to vacuuming to rodent control are important strategies to review, especially since in most circumstances, women are more likely to be exposed to these triggers. Important topics that need to be reviewed also include inhaler technique, early recognition of symptoms of worsening asthma, an action plan for acute asthma exacerbations, as well as an overview of how poorly controlled asthma can affect the pregnancy. Patients should also be provided with the opportunity to express their concerns and ask questions. In a multi-institutional prospective study, lower forced expiratory volume in 1 s (FEV1), but not asthma symptom frequency, was shown to be associated with adverse perinatal outcomes [9]. These data may be a reflection of the effect of asthma severity or poor asthma control on perinatal outcomes and emphasize the possibility of discrepancies between symptom-based assessment and more objective measurement of lung function in pregnant women with asthma. Finally, women with asthma need to receive the appropriate pharmacological treatment to achieve disease control. Population-based data do show that well-controlled asthmatics without exacerbations have better outcomes than women with exacerbations, but for obvious reasons, there are no randomized controlled trials evaluating this particular question. Although most clinical practices use symptom-based, guideline-directed assessments to decide on medication use, recent data from a randomized controlled trial suggest lower rates of exacerbation, improved quality of life, and reduced neonatal hospitalization when management decisions were based on measurements of exhaled nitric oxide in pregnancy [10]. It is likely that this improvement in outcomes is due to improved control, rather than the method of assessment itself.
Table 11.2 provides an overview of the asthma medications that are used in pregnancy. As in the nonpregnant population, the choice of pharmacological agent depends on disease severity. A frank discussion with the expectant mother and her partner should occur to encourage them to voice their concerns regarding asthma treatment in pregnancy. Most women are told to stop their inhalers at the time of pregnancy diagnosis because of FDA category listing. For that reason, a good amount of time should be spent on counseling about the use of asthma drugs in pregnancy. Explaining to women that asthma control is key to the health of the pregnancy and their baby is an important part of counseling and may have to be done repeatedly during the course of pregnancy. In general, most asthma medications are justifiable in pregnancy, and some have adequate safety data. As noted in Table 11.2, many of the drug choices are category C according to the FDA classification; however, these drugs are used routinely in the care of pregnant women with asthma. In addition, although leukotriene inhibitors are listed as category B, safety data are less reassuring than other drugs classified as category C. Omalizumab is classified as category B by the FDA despite the fact that all of the initial trials have excluded pregnant women. These safety data are based on animal studies which are limited by the fact that teratogenicity may be species specific. In addition, although prednisone may be associated with a small risk of cleft palate when administered in early pregnancy, the benefit of this drug in an acute exacerbation of asthma by far outweighs the small risk of malformation. Table 11.3 reviews the classification of asthma severity, which includes not only symptoms but also peak flow meter measurements.
Table 11.2
Overview of medications used in the treatment of asthma during pregnancy
Category | Best studied example | Risk category | Comment |
|---|---|---|---|
Short-acting ßeta 2-agonist | Albuterol | C | No clear risk of teratogenic effect on fetus. Specific birth defects have been reported but are not the same. These findings may be due to chance. Benefit outweighs risk |
Long-acting ßeta 2-agonist | Salmeterol | C | No clear risk of teratogenic effect on fetus. Benefit outweighs risk. Slightly more data available in salmeterol than formoterol |
Inhaled corticosteroids | Budesonide | B | Human experience reassuring. Infants should be monitored for hypoaldosteronism. Some recent data suggestive of increased risk of metabolic dysfunction in the offspring |
Anticholinergics | Ipratropium | B | Typically not used as primary agent but helpful in the treatment of acute exacerbations. Not expected to increase the risk of congenital malformations |
Methylxanthines | Theophylline | C | No human reports of teratogenicity. Requires monitoring levels due to increased clearance in the third trimester and significant drug interactions |
Cromoglycates | Cromolyn sodium | B | Not expected to increase the risk of congenital malformations. Human data reassuring |
Leukotriene modifiers | Montelukast | B | Safety data are limited in pregnancy. However, although congenital limb defects, no syndrome of malformations has been identified in relation to montelukast |
Systemic steroids | Prednisone/ methylprednisolone | C/D | Increased risk of growth abnormalities in animals, likely increased risk of cleft palates during first trimester exposures. Risk outweighs the risk in severe asthma |
Epinephrine | Terbutaline | B | Unlikely to increase the risk of birth defects |
Immunotherapies | Omalizumab | B | Limited human data. Risk benefit ratio needs to be considered. Pregnancy registry available |
Table 11.3
Classification of asthma severitya
Intermittent | Persistent | |||
|---|---|---|---|---|
Mild | Moderate | Severe | ||
Symptoms | ≤2 days/week | >2 days/week but not daily | Daily | Throughout the day |
Nighttime awakenings | ≤2×/month | 3–4×/month | >1×/week but not nightly | Often 7×/week |
Short-acting beta2-agonist use | ≤2 days/week | >2 days/week but not daily and not more than 1× on any day | Daily | Several times per day |
Interference with normal activity | None | Minor limitation | Some limitation | Significant limitation |
Peak Flow/ Pulmonary function tests | • Normal FEV1 • FEV1 > 80 % predicted • FEV1/FVC normal | • FEV1 ≥ 80 % predicted • FEV1/FVC normal | • FEV1 > 60 but <80 % predicted • FEV1/FVC decreased by 5 % | • FEV1 < 60 % predicted • FEV1/FVC decreased by > 5 % |
Other coexisting diseases may worsen asthma and may have to be treated in order to achieve optimal control. The most common of these disorders are allergic rhinitis, gastroesophageal reflux disease (GERD), sleep apnea, and psychiatric illnesses. Allergic rhinitis occurs in 80–90 % of nonpregnant asthmatics and worsens asthma symptoms. Management of the allergic rhinitis with drugs such as steroidal nasal sprays often improves asthma symptoms. Women who are pregnant can also develop a different form of rhinitis, called rhinitis of pregnancy. This typically occurs in the latter part of pregnancy and resolves completely within 2 weeks after delivery.
The prevalence of GERD among nonpregnant asthmatics varies between 30 and 90 %. In pregnant women with asthma, this number is likely higher given that GERD has been reported to be present in nearly 75 % of all pregnant women [11]. GERD can worsen bronchoconstriction via increased vagal tone, heightened bronchial reactivity, and microaspiration of gastric contents into the upper airway. Patients who have symptoms of GERD benefit from treatment. Although proton pump inhibitors are not expected to increase the risk of congenital malformation in experimental animal studies and limited human pregnancy exposures, ranitidine constitutes a safer first choice. Finally, asthma and psychiatric comorbidities may coexist. Stress and mental illness can worsen asthma in the pregnant women and may also complicate compliance.
During labor, the general management of asthma is not significantly different than above. Most patients with asthma do not require a labor and delivery plan. However, patients with more severe disease or those who suffered an exacerbation close to term would require a detailed plan. Stress dosing with steroids during labor can be considered in patients who have been on prolonged periods of systemic steroids during the pregnancy. Patients with active symptoms or more severe asthma may benefit from regional anesthesia. Epidural anesthesia reduces minute volume and oxygen consumption and may help prevent hyperinflation in patients with active symptoms and reduce oxygen consumption. If general anesthesia is to be considered, then ketamine and halogenated anesthetics are preferred. It is safe to use oxytocin and prostaglandin E2. However, ergotamine and ergot derivatives, 15-methyl prostaglandin F2 alpha, morphine, and meperidine should be avoided in pregnant women with asthma as they may be associated with an increased risk of bronchospasm.
Treatment of Acute Asthma Exacerbations
An overview of the management of acute asthma exacerbations in the pregnant woman is detailed in Fig. 11.2. More detailed information can be found in National Heart Lung and Blood Institute guidelines on asthma and pregnancy published in 2004. The treatment is similar to nonpregnant women with a few key differences that need to be highlighted. The first is to remember that during pregnancy, the normal PaCO2 is lower than in the nonpregnant state. Therefore, a normal or high PaCO2 heralds worsening respiratory failure and should be acted upon quickly. Second, hypoxia during asthma exacerbations can lead to fetal distress and decelerations. Therefore, immediate bronchodilators and supplemental oxygen should be administered. Finally, it should be noted that while the indications for airway intubation are the same in the pregnant asthmatic as the nonpregnant asthmatic, intubation during pregnancy, especially in the third trimester, can be more difficult. This is due to increased airway edema, low FRC and oxygen reserve, and a more profound response to sedatives from decreased venous return. Hence, the most experienced member of the team should perform the intubation and be familiar with difficult airway management procedures. Airway intubation is discussed in more detail in the critical care Chap. 2.
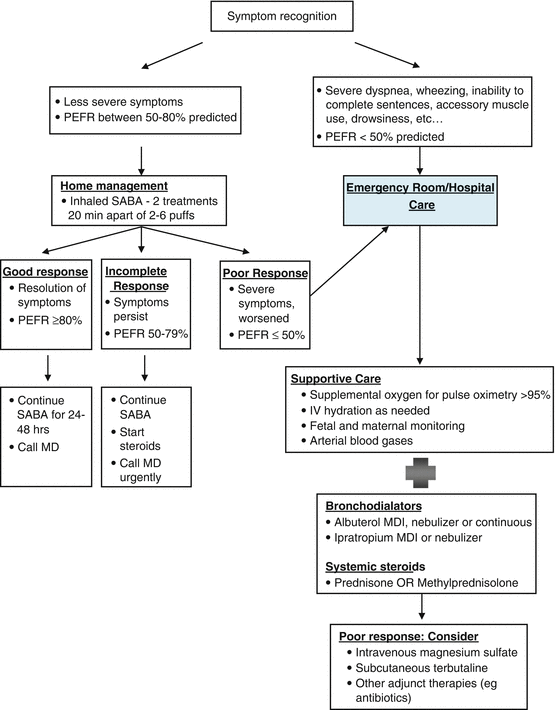

Fig. 11.2
Management of acute asthma exacerbation during pregnancy. Simplified from http://www.nhlbi.nih.gov/health/prof/lung/asthma/astpreg/astpreg_qr.pdf
Pneumonia in Pregnancy
Bacterial Pneumonia
Pneumonia is one of the leading causes of non-obstetric maternal deaths in the United States [12]. There are several categories of pneumonia based on the likely spectrum of pathogens: community-acquired pneumonia (CAP), healthcare-associated pneumonia, hospital-acquired pneumonia, and ventilator-associated pneumonia as well as pneumonia in the immune-compromised host. As pregnant women are usually young and healthy, CAP predominates.
Microbiology and Epidemiology
The overall rate of CAP in pregnant women is 0.5–1/1,000 pregnancies depending on the population being studied [13–15]. The risk of pneumonia is notably increased in gravidas with comorbid conditions such as asthma, anemia, and human immunodeficiency virus [16]. Tobacco and substance abuse have also been independently associated with an increased risk for pneumonia. Influenza increases the risk for development of bacterial pneumonia by denuding the respiratory epithelium and predisposing the host to infection.
In adults, the causative agents for CAP are identified in 40–60 % of cases when advanced testing techniques are utilized [17, 18]. The yield is much lower, in the range of 10–25 %, with regular testing. Though specific studies in pregnant women are lacking, the likely pathogens are not considered to be significantly different from those in the general population. Streptococcus pneumoniae is the most common single pathogen isolated in 30–50 % followed by Haemophilus influenzae and Mycoplasma pneumonia [19]. Pregnant women may be more likely to contract viral infections and tend to have more severe disease than the nonpregnant population. Therefore, the estimates above may be somewhat different in pregnancy.
Effect of Pregnancy on the Disease
Gingival hyperplasia in pregnancy may promote changes in oral flora and promote growth of anaerobic bacteria. Aspiration risk and heartburn [11] may be increased in pregnancy, especially when undergoing sedative procedures or general anesthesia. Whether these changes and increased gastroesophageal reflux disorders are associated with increased risk of pneumonia is not clear. Immune alterations in pregnancy that promote maternal tolerance to the fetus may impair optimal function of host defense mechanisms and increase the risk of infections. Pregnant women have decreased lung capacity and decreased ERV and RV resulting in a reduction in functional residual capacity. A state of compensated respiratory alkalosis is established by increasing minute ventilation. This is largely secondary to an increase in tidal volume and to a lesser extent an increase in respiratory rate. Healthy gravid subjects have increased cardiac output and decreased oncotic pressure which peaks in the third trimester that promotes transudation of fluid into the pulmonary interstitium. These changes diminish oxygen reserve, increase the risk of development of pulmonary edema with fluid resuscitation, and predispose to respiratory failure and predispose women to more severe disease.
Effect of Disease on the Pregnancy
Pneumonia may be complicated by hypoxia, respiratory failure, or death, and preterm delivery appears to be the most common obstetric complication associated with maternal pneumonia. While intrauterine infection is known to cause preterm delivery, a causal relationship between pneumonia in pregnancy and preterm delivery is not well established. It is possible that higher levels of cytokines and other mediators such as TNF-α and prostaglandin F2 reported in bacterial infections may lead to preterm delivery and low birth weight. Other reported complications include placental abruption, preeclampsia and eclampsia, and low Apgar scores [20–22]. It is unclear, however, whether these complications are related to the actual infection or to other host factors.
Differential Diagnosis
Common causes for respiratory distress in pregnancy include infection such as urinary tract infection, pulmonary edema, asthma, aspiration, and pulmonary embolus.
Diagnostic Evaluation
The clinical spectra of pneumonia caused by different pathogens overlap considerably. Thorough history and examination along with microscopic examination of respiratory secretions may narrow the differential diagnosis and identify the offending pathogen. Urine pneumococcal and legionella antigen may also aid in guiding antibiotic therapy and should be considered for patients requiring admission. During influenza seasons, respiratory viral panel should be sent. Though blood cultures are usually negative and of low yield, they may add value in the patient requiring admission to the intensive care unit (ICU). Arterial blood gas should be done for all patients with hypoxia or those requiring admission to the ICU and interpreted according to pregnant status.
Chest X-ray should be performed in patients suspected of having pneumonia and helps confirm the diagnosis or show evidence of a complicated pneumonia such as lung abscess or pleural effusion. Computed tomography scan is unlikely to add value in the management of pneumonia, unless empyema is suspected. Ultrasound guidance likely reduces the risk of complications with thoracentesis in pregnancy given the cranial displacement of the diaphragm in pregnancy. Bronchoscopy though rarely needed can be performed safely in pregnancy and should not be withheld when indicated.
Management/Treatment
Supportive Treatment
General supportive measures are similar in patients with various types of pneumonia. For patients with a viable fetus who require admission, the obstetric team should be consulted for fetal monitoring as well as timing of delivery in the event of fetal distress. Hypoxia, acidosis, and fever should not be tolerated as they are independently associated with poor fetal outcomes. Oxygen should be supplemented for goal saturations > 95 % or PaO2 above 70. Fever should be treated aggressively for a goal temp of less than 38 °C.
In cases of severe pneumonia associated with respiratory failure, early intubation should be considered. Intubations in pregnancy have a higher failure rate than the general surgical population (see Chap. 2 on airway intubation). Attempts to maintain CO2 within an acceptable range may be challenging in the event of acute respiratory distress syndrome (ARDS) and the use of lung protective strategies. Low tidal volume ventilation strategy with a target tidal volume of 6 ml/kg is recommended for ARDS [23]. Though pregnant women were excluded in the acute respiratory distress network studies on lung protective strategies, low tidal volume ventilation should be attempted, initially with a higher respiratory rate to maintain ventilation given the survival benefit observed in the nonpregnant population. However, higher tidal volumes may be required to correct acidosis that may compromise the fetus, in such instances attempts should still be made to keep the plateau pressure below 30 cm of water as barotrauma is thought to contribute significantly to lung injury. PaCO2 levels need to be watched closely, and given the 10 mmHg gradient between fetal and maternal, maternal PaCO2 should be kept at 55 mmHg or lower. Use of bicarbonate to correct the PH has been suggested in the nonpregnant population though clinical studies to support this approach are limited. It is thought that the transfer of bicarbonate across the placenta is slow and may not be adequate to correct fetal acidosis. While the decision to admit patients to the ICU is complex and should be individualized, clinicians should have a lower threshold when evaluating pregnant mothers.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


