Endometrial histology with and without progestin in HRT. Images reproduced with the permission of Professor ARE Williams, University of Edinburgh. (a) Unopposed estrogen effect – disordered proliferative endometrial pattern. The endometrial glands have an irregular architectural pattern, but are distributed evenly throughout the stroma, without significant crowding, and with no increase from normal in the gland–stroma ratio. (b) Simple non-atypical endometrial hyperplasia. The endometrial glands mostly have a simple tubular architecture, some showing cystic dilatation. The epithelium is active in appearance, composed of tall columnar cells in which mitotic activity is present (not seen at this magnification). There is an increase in gland–stroma ratio. (c) Secretory differentiation in HRT effect. Glands are widely dispersed amongst endometrial stroma showing confluent decidual change. The glandular epithelium is mostly inactive, but some glands show obvious secretory differentiation with vacuolation of cytoplasm and a tortuous architecture, reminiscent of the mid-secretory phase. There is a decrease in the gland–stroma ratio. (d) Atrophic changes of continuous combined HRT. Glands show a flattened inactive appearance, and are widely dispersed in stroma showing confluent decidual change.
Estrogen/progestin regimes in HRT
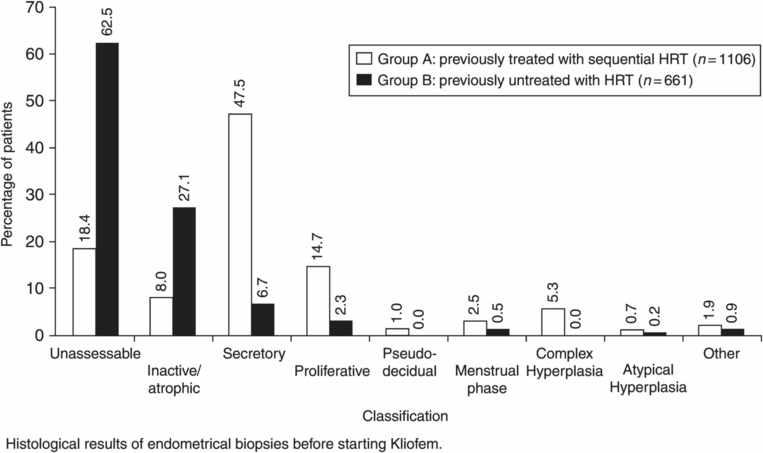
The first estrogen/progestin regimens to be developed were sequential and today these are usually designed to provide 28-day cycles involving continuous estrogen and with progestin added for 12–14 days. On such regimens most women bleed for a few days from near the end of the progestin phase, but in any one cycle some women will not bleed and a small proportion of women are consistently free of bleeding. The absence of bleeding is not thought to indicate failure of endometrial protection. Such regimens are suitable for women both in the perimenopause requiring HRT and in the established menopause. The more recently introduced continuous combined estrogen/progestin regimens aim at achieving freedom from bleeding for a majority of women. On the standard regimens of this form around 80% of women become bleed-free, though a proportion will experience irregular bleeding, especially in the initial months. These regimens are not suitable before the menopause is established. It is expected that a majority of women on continuous combined regimens will have atrophic endometrium. Figures 16.2 and 16.3 illustrate the diversity of endometrial histology seen in untreated women and women using a wide range of sequential HRT preparations, all biopsied before starting on a continuous combined HRT preparation, Kliofem (Figure 16.2), or biopsied after 9 months’ Kliofem (Figure 16.3) [5]. In this study the endometrial biopsies labeled as unassessable were taken to be additional cases of inactive/atrophic endometrium. The study illustrates the diversity of endometrial histology in postmenopausal women whether using HRT or not. The most consistent pattern appears to be in women using continuous combined HRT which may correlate with the evidence that this form of HRT appears to reduce endometrial cancer risk below the background rate.
Endometrial histology in women treated with sequential estrogen/progestin HRT and untreated women.

Endometrial histology in women treated with continuous combined estrogen/progestin HRT in women previously untreated or previously treated with a sequential HRT regimen.
Mammographic breast density
In untreated women increased breast density is important, both because increased density on mammography may mask small lesions and because there is a suggested association between increased breast density and breast cancer risk. It is known that estrogen/progestin HRT regimens increase breast density. It is not proved that this necessarily raises breast cancer risk but it may increase the difficulty in detecting lesions. Studies that compare sequential and continuous combined regimens suggest that the increase in breast density is more marked with continuous combined regimens. Currently the issue of mammographic breast density is attracting considerable attention in the USA where several states have introduced legislation concerning the responsibility of doctors to communicate information about increased breast density to women. A Swedish study of mammographic breast density reported that compared with untreated women, the risk ratio for increased density in sequential estrogen/progestin HRT was 3.6 (95% Cl 1.6–7.7) whereas with continuous combined HRT the risk ratio was 12.4 (95% CI 6.3–24.4). The rates were even higher if only women over 50 years were considered [6]. In the light of this it is of interest that the European Prospective Investigation into Cancer and Nutrition reported that overall the risk ratio for higher risk with continuous combined HRT compared with sequential HRT was 1.43 (95% CI 1.19–1.72). In their analysis, country by country, they observed the point estimate for breast cancer risk to be higher in association with continuous combined HRT regimens than with sequential HRT regimens in Denmark, France, Germany and Norway whereas the reverse was found for British women [7].
Progestins and safety – estimating the influence of progestins in HRT from trials
Progestogens have been accepted as an essential safety component of any HRT regimen where there has not been a hysterectomy. By including a progestogen in HRT the regimens facilitate more predictable scheduling of monthly bleeding, or even the possibility of freedom from bleeding for many women if the progestogen is given continuously. Unfortunately the additional actions of progestogens beyond the specific endometrial target introduces the potential for effects that might affect risk in the long term. This potential contribution to any long-term risk associated with HRT is ideally explored in appropriate clinical trials [8, 9].
In the past 20 years there has been a growing emphasis on the difficult task of studying the effects of HRT in large randomized controlled trials. The results of the small number of these major trials that have been carried out will be addressed elsewhere in this book (cancer in Chapter 22, and venous thromboembosis in Chapter 23) but an aspect directly relevant to this chapter is where these trials provide insight into the contribution of progestin in HRT. This was highlighted, in particular, by the reports from the Women’s Health Initiative (WHI) trials in American women where some adverse outcomes appeared more common if a progestin was included in the HRT. It is important that the effects of adding progestin to HRT are discussed here but it must be emphasized that although there is discussion about its possible negative impact, there is no strong voice proposing that women who have a uterus would do better using HRT without progestogen. However there has undoubtedly been a trend towards exploring lower-dose estrogen and progestin HRT preparations with a view to maximizing safety.
It is a reflection of the predominance of continuous combined regimens after the menopause that the landmark WHI trial opted for a continuous combined estrogen/progestin regimen (conjugated equine estrogens + medroxyprogesterone acetate) for women who required uterine protection. These trials will have been described elsewhere in this book but here it is necessary to emphasize that the key difference between the two trials was that the larger trial compared this estrogen/progestin HRT regimen against placebo whereas the other WHI trial in women who had hysterectomy compared estrogen-only HRT (conjugated equine estrogens) against placebo. In this chapter the two trials are referred to as the E+P trial and the E-only trial respectively. Comparison of the women in the two trials revealed that the hysterectomy E-only trial population was “more racially diverse, had less favorable cardiovascular risk profiles and more commonly had oophorectomy and prior hormone use” [8].
There has been considerable interest in the differences in outcomes between these two WHI trials, and the specific therapeutic difference is involvement of progestin in the E+P study. Now more than 10 years later we have the major WHI publication that provides a “comprehensive integrated overview of findings from the two Women’s Health Initiative hormone therapy trials with extended post-intervention follow-up” [8]. In focusing on this it is important to express some caveats. Firstly that a single progestin, medroxyprogesterone acetate, was used in these trials and that we do not have this weight of evidence concerning other progestins. Secondly it is important to note that because the intention of WHI was to look at health outcomes in HRT use in older women it is not straightforward to transpose the outcomes of a 50–79-year-old population to the majority of likely users of HRT who are largely under 60 years of age. On the other hand, though with less statistical power than the global studies, WHI has provided the outcomes for the 50–59-year-old subgroup. In addition to that major randomized controlled trial we now also have the data from the Danish Osteoporosis Study which focused on women below 60 years of age and which provided extended treatment and follow-up data on women randomized to use a sequential estradiol/norethisterone regimen or placebo [9].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


