Chapter 16
Problems of Early Pregnancy
Katherine W. Arendt MD
Chapter Outline
PHYSIOLOGIC CHANGES OF EARLY PREGNANCY
ABORTION AND INTRAUTERINE FETAL DEMISE
CERVICAL INSUFFICIENCY OR INCOMPETENCE
GESTATIONAL TROPHOBLASTIC DISEASE
Obstetric disease of early pregnancy may result in significant maternal morbidity and even mortality. Safe care of patients with obstetric disease involves a thorough understanding of the physiologic changes of early pregnancy as well as the specific issues associated with each pathologic condition.
Physiologic Changes of Early Pregnancy
Respiratory System
The respiratory system undergoes profound physiologic changes during early pregnancy. Increased progesterone concentration stimulates respiratory efforts by increasing the sensitivity of the respiratory center to carbon dioxide. Minute ventilation increases by at least 15% by 12 weeks’ gestation and by 25% by 20 weeks’ gestation. This results from an increase in tidal volume (respiratory rate is unchanged) and exceeds the increase in oxygen consumption. The result is a respiratory alkalosis with maternal arterial partial pressure of carbon dioxide decreasing to 30 to 33 mm Hg by 10 to 12 weeks’ gestation. Moreover, maternal arterial partial pressure of oxygen increases to 106 to 108 mm Hg in the first trimester. Decreased bicarbonate concentration partially compensates for the modest respiratory alkalosis that results from the physiologic hyperventilation, leading to a maternal pH that is slightly above normal (i.e., approximately 7.44). There is little or no change in lung capacities during the first half of pregnancy. Women in early pregnancy who undergo mechanical ventilation require increased minute ventilation.
Cardiovascular System
The cardiovascular system also undergoes profound changes early in pregnancy. Cardiac output increases 20% to 25% by 8 weeks’ gestation and 35% to 40% by 20 weeks’ gestation. Systemic vascular resistance decreases 30% by 8 weeks’ gestation. Maternal mean arterial pressure decreases approximately 6 mm Hg at 16 to 24 weeks’ gestation and returns to normal near term.
Aortocaval compression typically occurs after 18 to 20 weeks’ gestation, when the uterine fundus reaches the umbilicus and is large enough to compress the aorta and vena cava when the patient is supine. Left uterine displacement is rarely needed in early pregnancy, but when the uterine size is equivalent to an 18- to 20-week gestation, left uterine displacement should be attained by elevating the right hip 15 degrees off midline with a wedge or blankets. The need for left uterine displacement occurs earlier in gestation in the presence of multiple gestation, polyhydramnios, or gestational trophoblastic disease.
Blood volume increases throughout pregnancy; the average prepregnancy blood volume of 4350 mL (76 mL/kg) increases to 4700 mL (81 mL/kg) at 12 weeks’ gestation, to 5500 mL (89 mL/kg) at 20 weeks’ gestation, and to approximately 6600 mL (97 mL/kg) at term. The increase in blood volume is primarily the result of greater plasma volume because red blood cell volume increases to a smaller degree (27 mL/kg). Because pregnant women have an expanded blood volume, they typically tolerate a blood loss of 500 to 1500 mL during the first half of pregnancy. A blood loss of 500 to 1500 mL rarely requires blood transfusion, provided that the blood loss is replaced with an adequate volume of crystalloid or colloid.
Gastrointestinal System
An increased progesterone level causes relaxation of lower esophageal sphincter tone as early as the first trimester. Fasting gastric volume is approximately 30 mL in both nonpregnant women and women in early pregnancy. Metoclopramide 10 mg, administered intravenously 15 to 30 minutes before anesthesia, can reduce this volume by 50%.1 In a study of 100 pregnant women undergoing general anesthesia by mask at 6 to 22 weeks’ gestation, a pH electrode showed reflux of gastric contents into the esophagus in 17% of patients.2 Most episodes of reflux occurred in patients who experienced hiccups. Only 2% had regurgitation of gastric contents into the pharynx, and no patient demonstrated clinical evidence of pulmonary aspiration.
General anesthesia may be safely administered by means of a mask or a laryngeal mask airway by experienced anesthesia providers in selected obstetric patients during early pregnancy. Many anesthesia providers are comfortable managing an airway without tracheal intubation until 18 to 20 weeks’ gestation, when the uterus moves out of the pelvis. The latter movement leads to anatomic and intragastric pressure changes that predispose to gastroesophageal reflux. Some anesthesia providers prefer to intubate the trachea of pregnant women who require general anesthesia as early as 12 to 14 weeks’ gestation, given that hormonal changes leading to sphincter relaxation are present early in pregnancy. Patients who receive general anesthesia during the first half of pregnancy should be intubated if they are at increased risk for gastric content aspiration (e.g., history of gastroesophageal reflux, morbid obesity, food ingestion within 6 to 8 hours). Pharmacologic prophylaxis (e.g., sodium citrate, a histamine-2 (H2) receptor antagonist, and/or metoclopramide) is likely to further reduce the risk for aspiration pneumonia (see Chapter 29). Neuraxial anesthesia is associated with a lower risk for aspiration than general anesthesia.
Nervous System
During early pregnancy, the nervous system is more sensitive to general and local anesthetic agents. The minimum alveolar concentration (MAC) for volatile anesthetic agents is decreased by approximately 30%, although the underlying mechanism for this change is unclear. A recent study that compared patients undergoing cesarean delivery with nonpregnant patients undergoing elective gynecologic surgery found no difference between groups in electroencephalographic measures during general anesthesia with similar end-tidal concentrations of sevoflurane.3 Because it is well-proven that MAC decreases in pregnancy, this study implies that MAC in pregnant women may not correlate well with depth of anesthesia. Further research is needed in this area.
Ectopic Pregnancy
Ectopic pregnancy occurs when the fertilized ovum implants outside the endometrial lining of the uterus. Death, infertility, and recurrent ectopic pregnancy are possible sequelae. The frequency of ectopic pregnancy in the United States increased fourfold to fivefold between 1970 and 1992 but appears to have stabilized at a rate of approximately 16 per 1000 pregnancies.4,5 A higher prevalence of associated risk factors, especially pelvic inflammatory disease, as well as earlier diagnosis of previously unrecognized ectopic pregnancies may account for the reported increase.
Ruptured ectopic pregnancy is a leading cause of pregnancy-related maternal death during the first trimester and accounts for 6% of all pregnancy-related maternal deaths in the United States.6,7 Most deaths result from hemorrhage (93%); infection (2.5%), embolism (2.1%), and anesthetic complications (1.3%) are less common causes.8 More than 30% of women who have had an ectopic pregnancy subsequently suffer from infertility, and 5% to 23% have a second ectopic pregnancy.9
The number of deaths from ectopic pregnancy decreased in the United States from 1970 through 2007. The case-fatality rate decreased from 35.5 deaths per 10,000 ectopic pregnancies in 1970 to 3.8 per 10,000 in 1989,10 and the ectopic pregnancy mortality ratio decreased from 1.15 deaths per 100,000 live births in 1980 through 1984 to 0.5 death per 100,000 live births in 2003 through 2007.11 The U.S. Centers for Disease Control and Prevention attributes this decline to “improvements in the sensitivity, accuracy, and use of pregnancy testing, ultrasound for diagnosis, and improvements in therapeutic modalities, including laparoscopic surgery and medical management of ectopic pregnancy.”12 However, a recent cluster of 11 maternal deaths from ectopic pregnancy in Florida between 2009 and 2010 increased Florida’s ectopic pregnancy mortality ratio from 0.6 death per 100,000 live births in 1999 through 2008 to 2.5 deaths per 100,000 live births in 2009 through 2010.12 Because these women collapsed (likely from acute rupture and hemorrhage) without ever seeking health care, it was believed that limited access to early care may have contributed to the adverse outcomes. Further, of the 11 women who died, 6 tested positive for illicit drugs at autopsy. Ectopic pregnancy deaths historically have been more common in teenagers10 and are 3 to 18 times higher in African-American women than in white women.6,8,10
Factors that alter the normal fallopian tube transport system for the fertilized ovum increase the risk for ectopic pregnancy. These factors include (1) previous ectopic pregnancy; (2) previous tubal surgery; (3) pelvic inflammation, especially infection with Chlamydia trachomatis; (4) congenital anatomic distortion such as that caused by exposure to diethylstilbestrol in utero; (5) previous pelvic or abdominal surgery; (6) use of a contraceptive intrauterine device, which may be associated with interstitial ectopic pregnancy; (7) delayed ovulation; (8) hormonal changes associated with ovulation induction or progestin-only oral contraceptives; (9) lifestyle factors (e.g., smoking, vaginal douching); (10) history of infertility; and (11) assisted reproductive technology (ART) procedures (e.g., zygote transfer into the fallopian tube or uterine cavity).13 However, one third of patients with ectopic pregnancies have no identifiable risk factors.
The fertilized ovum can implant anywhere along the path of migration or in the abdominal cavity (Figure 16-1). Most ectopic pregnancies (98%) are tubal (infundibular or fimbrial, 6%; ampullary, 78%; isthmic, 12%; interstitial or cornual, 2%). The remaining 2% implant on the cervix, vagina, or ovary or elsewhere in the abdomen.14 An increasing number of cesarean scar ectopic pregnancies, which may be on a continuum with early placenta accreta, are being reported.
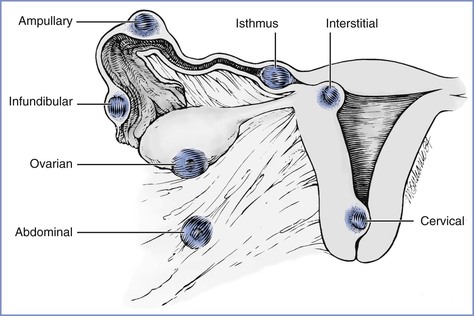
FIGURE 16-1 Potential locations of ectopic pregnancies. The majority occur in the ampullary portion of the fallopian tube. (Reprinted from Chantigian RC, Chantigian PDM. Problems in early pregnancy. In Chestnut DH, Polley LS, Tsen LC, Wong CA, editors. Chestnut’s Obstetric Anesthesia. 4th edition. Philadelphia, Mosby, 2009. Modified from DeCherney AH, Seifer DB. Ectopic pregnancy. In Gabbe SG, Niebyl JR, Simpson JL, editors. Obstetrics: Normal and Problem Pregnancies. 2nd edition. New York, Churchill Livingstone, 1991:811.)
In patients who undergo ART procedures, ectopic pregnancies have been reported in approximately 2% of pregnancies.13 Most of these pregnancies are tubal; however, approximately 6% are ovarian, abdominal, or cervical, and 12% to 15% are heterotopic (see later discussion).14
Clinical Presentation
The clinical presentation of the patient with an ectopic pregnancy depends on the gestational age, site of implantation, and extent of hemorrhage. Prior to rupture, the signs and symptoms are often subtle. Classic clinical signs of impending rupture or a ruptured tubal pregnancy include abdominal or pelvic pain (95%), delayed menses (75% to 95%), and vaginal bleeding (60% to 80%). Vaginal bleeding results from the breakdown and shedding of the decidual lining of the uterine wall, which is probably associated with decreased hormone production by the corpus luteum and inadequate human chorionic gonadotropin (hCG) production by the ectopic trophoblast. Pain often precedes vaginal bleeding. Patients with hemorrhage (with or without tubal rupture) may experience dizziness or syncope, may have the urge to defecate because of the effect of blood in the cul-de-sac, and may have shoulder pain from diaphragmatic irritation by intra-abdominal blood.
Physical findings include abdominal tenderness with or without rebound (80% to 95%), a uterus that is smaller than expected for dates (30%), and a tender adnexal mass (30% to 50%). A bulging cul-de-sac suggests hemoperitoneum. With significant hemorrhage there may be signs of shock, but some patients may appear hemodynamically stable despite a hemoperitoneum volume of 1000 to 1500 mL; presumably, these patients have an ectopic pregnancy with slow bleeding and are able to compensate for the gradual blood loss.
Diagnosis
Ectopic pregnancy should be excluded in any patient who has pelvic pain and a positive pregnancy test. In a woman of reproductive age, the symptoms of ectopic pregnancy must be differentiated from (1) a threatened, inevitable, or incomplete abortion; (2) infection after attempted abortion; (3) pelvic inflammatory disease; (4) a degenerating fibroid; (5) appendicitis and other gastrointestinal diseases; (6) ovarian torsion; (7) a ruptured or bleeding ovarian cyst; (8) a trapped retroverted uterus in pregnancy; and (9) nephrolithiasis.
Current tests allow early diagnosis of ectopic pregnancy and prompt treatment that decreases morbidity and mortality.9 Diagnostic algorithms include the following guidelines:
1. Ultrasonography can reliably confirm only the presence of an intrauterine pregnancy. The ectopic pregnancy itself may be difficult to visualize.15 Transvaginal ultrasonography is the current modality of choice because it can detect an intrauterine gestational sac as soon as 21 days after conception (when the beta-hCG concentration is greater than 1400 mIU/mL with use of the International Reference Preparation [IRP] standard). Transabdominal ultrasonography can visualize an intrauterine pregnancy when the serum beta-hCG concentration is higher than 6000 to 6500 mIU/mL IRP.16
2. A serial beta-hCG concentration that decreases, plateaus, or shows a subnormal increase (< 53% over 48 hours) usually indicates a nonviable pregnancy—either an ectopic pregnancy or an impending abortion.17 With a spontaneous abortion, a decline in beta-hCG concentration of at least 21% to 35% should be seen over 2 days. A slower decline is suggestive of an ectopic pregnancy. A beta-hCG concentration greater than 100,000 mIU/mL is usually associated with a viable intrauterine pregnancy.18
3. A serum progesterone concentration greater than 25 ng/mL is usually associated with a viable pregnancy. A concentration less than or equal to 5 ng/mL usually indicates a nonviable pregnancy but cannot distinguish a spontaneous abortion from an ectopic pregnancy.19 Most ectopic pregnancies are associated with progesterone levels between 5 and 25 ng/mL, a fact that limits the usefulness of this test.
In the past, culdocentesis was used to aid in the diagnosis of hemoperitoneum and ectopic pregnancy. Although a positive result is highly predictive of hemoperitoneum, the advent of pelvic ultrasonography and rapid quantitative beta-hCG tests limits its value in the diagnosis of ectopic pregnancy.
Obstetric Management
Management options for ectopic pregnancy are expectant, medical, and surgical. Management choice depends on the symptoms and diagnostic findings.
Expectant management may be used for selected asymptomatic patients with early tubal ectopic pregnancies and stable or decreasing beta-hCG levels. Successful resolution has been reported in approximately 50% of these selected patients.4 If expectant management is unsuccessful, a medical or surgical approach is required.
The American College of Obstetricians and Gynecologists (ACOG) as well as the American Society of Reproductive Medicine have published guidelines for the medical management of ectopic pregnancy.20,21 Systemic, intramuscular, oral, and intragestational forms of chemotherapy have been used successfully in the medical management of ectopic pregnancy. Methotrexate, a folate antagonist that interrupts DNA synthesis and thus cell replication, inhibits growth of trophoblastic cells of the placenta and is commonly used to treat ectopic pregnancy. Because methotrexate is toxic to all rapidly-dividing tissues of the body, there are many contraindications to medical treatment of ectopic pregnancy, including immunodeficiency and pulmonary, liver, renal, or hematologic disease. Further, the ACOG has recommended that only early tubal pregnancies (i.e., no cardiac activity, a gestational sac with a diameter < 3.5 to 4.0 cm, and no evidence of tubal rupture or hemoperitoneum) be treated with methotrexate.
Methotrexate treatment protocols include a single-dose regimen, a two-dose regimen, and a fixed multidose regimen; the multidose regimen is reserved for patients with high beta-hCG levels (i.e., > 5000 mIU/mL). From day 4 to day 7 after methotrexate treatment, a decrease in beta-hCG level of 15% must be present to consider the treatment successful. Otherwise, repeat methotrexate treatment or surgical intervention is required. Follow up and close monitoring until beta-hCG level reaches nonpregnant values is imperative because of the risk for rupture and hemorrhage. Side effects of methotrexate can be severe and include abdominal pain, vomiting, stomatitis, severe neutropenia, and pneumonitis. Compared with surgical management, medical management of ectopic pregnancy provides no difference in overall tubal preservation, tubal patency, risk for repeat ectopic pregnancy, or success of future pregnancies.
Surgical management depends on the location of the pregnancy, the hemodynamic stability of the patient, the available equipment, and the surgeon’s expertise. Most often, diagnostic laparoscopy is performed to confirm the diagnosis and locate the ectopic pregnancy. For tubal ectopic pregnancies, a salpingostomy, salpingotomy, or salpingectomy (usually partial) is performed by means of laparoscopy or laparotomy. To aid hemostasis during laparoscopic removal of the ectopic pregnancy, some obstetricians inject dilute vasopressin into the surface of the fallopian tube. This agent causes marked blanching of the tube and results in a relatively bloodless surgical field. If the vasopressin is accidentally injected intravenously, a marked increase in maternal blood pressure may occur.
A laparotomy is indicated if the surgeon is not trained in operative laparoscopy, laparoscopic removal is anticipated to be difficult (e.g., tube diameter > 6 cm or an interstitial location of the ectopic pregnancy), or there is uncontrollable bleeding. Laparotomy should be performed immediately if there is hemodynamic instability; these cases often require a partial or total salpingectomy. If a partial salpingectomy is performed, tubal repair may be performed primarily or during a second operation. Although some experts have noted that outcomes from randomized trials comparing salpingostomy and salpingectomy are lacking,22 the risk for persistent ectopic pregnancy may be higher after salpingostomy than after salpingectomy.23
Interstitial, cervical, cesarean scar, and abdominal ectopic pregnancies as well as early placenta accreta may present significant diagnostic and therapeutic challenges, resulting in delay of diagnosis and treatment. There is potential for massive hemorrhage because of disruption of organs and adjacent tissues. The desire to preserve fertility may result in greater blood loss as tissue and organ preservation are attempted.
Interstitial pregnancy often goes unrecognized and may manifest as uterine wall rupture, massive hemorrhage, and shock. Conservative surgery (e.g., cornual resection) may be attempted, but hysterectomy may be required if uterine damage is severe.
Cervical pregnancy often results in massive hemorrhage because of the inability of the cervix to contract. In the past, most cervical pregnancies necessitated hysterectomy to control hemorrhage. More current management options that have greater likelihood of maintaining fertility include (1) methotrexate therapy, (2) local excision, (3) cerclage and tamponade, (4) ligation of the hypogastric arteries or the cervical branches of the uterine arteries, and (5) angiographic embolization of the uterine arteries followed by a dilation and evacuation (D and E) procedure (see later discussion).24
Cesarean scar pregnancy occurs when a gestational sac implants in the uterine scar defect (niche) at the site of a previous cesarean delivery. Cesarean scar pregnancy has a high complication rate. Although relatively rare, its incidence is rising with increasing cesarean delivery rates and currently may be as high as 1 in 1800 pregnancies.25,26 Jurkovic et al.26 described two types of cesarean scar pregnancies: (1) implantation on the scar with enlargement into the uterine cavity, and (2) implantation into a scar defect with growth into the myometrium. Depending on its progression, the former type may grow normally or may be treated medically. Scar implantation results in an increased risk for hemorrhage at delivery. Growth into the myometrium may lead to eventual rupture and bleeding in the first trimester; prompt surgical intervention is preferred over medical management in this situation.
In a review of 112 cases of cesarean scar pregnancies, Rotas et al.25 found that approximately half occurred in women with only one previous cesarean delivery. Many patients have vaginal bleeding, abdominal cramps, and/or lower abdominal pain. Up to one third of cases may be asymptomatic and are diagnosed during routine ultrasonography. A review of 751 published cases of cesarean scar pregnancy found that the diagnosis was missed in 107 of 751 cases (13.6%).27 Transvaginal ultrasonography was the best diagnostic tool. There were a total of 31 different primary treatment approaches, which included hysterectomy, dilation and curettage (D and C), hysteroscopic excision, uterine artery embolization, and intra-gestational aspiration or injection of methotrexate or potassium. Complications occurred in 331 of the 751 cases (44.1%), of which the most common was hemorrhage. The authors noted that local methotrexate and hysteroscopic-directed procedures had the lowest complication rates and that curettage, systemic methotrexate therapy, or embolization as single treatments should be avoided.
The incidence of early placenta accreta is also rising as a result of increasing cesarean delivery rates. It is defined as penetration of the placenta into the myometrium, which is discovered in the first or early second trimester. Because of similarities in pathogenesis, it is thought—although not confirmed—that early placenta accreta may develop from cesarean scar pregnancy. A recent review found that 15 of 47 (32%) patients with early placenta accreta had spontaneous uterine rupture, in most cases followed by bleeding and shock, which resulted in laparotomy, hysterectomy, or uterine artery embolization.27 Although the gestational age is early, it is imperative that the anesthesia team is aware of the risk for hemorrhage during surgical intervention for cesarean scar pregnancy and early placenta accreta.
Abdominal pregnancy is defined as implantation in the peritoneal cavity, not including the fallopian tubes, ovaries, or ligaments, and is associated with a high incidence of maternal morbidity and fetal demise.28 In a recently published series of advanced extrauterine pregnancies, Worley et al.29 identified ten women who presented with extrauterine pregnancies beyond 18 weeks’ gestation, of whom three met the diagnostic criteria for abdominal pregnancy. All patients had difficult surgery, nine patients required blood transfusion, and only five fetuses survived after complicated courses.
Diagnosis of abdominal pregnancy can be difficult, historically being missed in as many as one of nine cases.28 The diagnosis was missed prior to delivery in four of the ten cases in the series of Worley et al.29 Abdominal pain, vaginal bleeding, symptoms consistent with partial bowel obstruction, shock, or death may be the first indication of this unusual type of pregnancy. Ultrasonography is useful but may miss the diagnosis in more than 50% of cases. Magnetic resonance imaging may prove to be a more sensitive diagnostic tool.
If an extrauterine pregnancy is suspected in early gestation, laparoscopy can be used to diagnose and remove gestational products. If the extrauterine pregnancy is not identified until late gestation, it is associated with decreased placental perfusion (which typically results in fetal growth restriction) and oligohydramnios (which often results in pulmonary hypoplasia and anatomic deformities). In 1993, Stevens30 reviewed published cases of abdominal pregnancy since 1809 and found that 63% of infants survived when born after 30 weeks’ gestation.
Management of an advanced extrauterine pregnancy consists of laparotomy and delivery of the fetus. Once the fetus is delivered, management of the placenta is controversial and fraught with hazard. Removal of the placenta is associated with massive hemorrhage, prolonged and complicated surgery (e.g., bowel resection), and an increased risk for maternal mortality. A decision to leave the placenta in situ results in a higher risk for infectious morbidity as well as a potential greater need for additional surgery.30,31 In the series of Worley et al.,29 the placenta was left in situ in two patients, both of whom developed serious complications. The site of placental implantation and the ability to adequately ligate the blood supply often dictates the obstetrician’s decision about management of the placenta.
Heterotopic pregnancy describes the simultaneous occurrence of an ectopic and an intrauterine pregnancy. Historically, it was thought to occur in 1 in 30,000 spontaneous pregnancies.32 However, in patients undergoing ART, 0.2% to 3% of pregnancies may be heterotopic.14,33 Difficulty visualizing the entire fallopian tube on ultrasonography, combined with normal or slightly elevated beta-hCG measurements (i.e., low serum levels from the ectopic pregnancy combined with normal levels from the intrauterine pregnancy), make the early diagnosis of heterotopic pregnancy difficult.34 This diagnosis should be suspected in cases in which clinical signs of an ectopic pregnancy and a confirmed intrauterine pregnancy coexist. In most cases, the ectopic pregnancy is removed surgically, which can be difficult when trying to maintain the intrauterine pregnancy. Alternatively, transvaginal ultrasonography–guided injection of potassium chloride into the ectopic pregnancy has been performed successfully; however, as many as 55% of patients may require subsequent surgery.35 The patient often sustains the normal intrauterine pregnancy to term.
Patients with ectopic pregnancies who are Rh-negative should receive Rh0(D) immune globulin.36
Anesthetic Management
Patients with an unruptured tubal pregnancy usually have normal intravascular volume, minimal bleeding before and during surgery, and low anesthetic and surgical risk. Anesthetic considerations for laparoscopy or laparotomy are summarized in Box 16-1. Although most patients may prefer general anesthesia, neuraxial anesthesia with an upper sensory level to at least T4 may be an alternative in selected patients. Shoulder pain from diaphragmatic irritation may occur and can be treated with intravenous analgesics (e.g., fentanyl 1 to 2 µg/kg).
A ruptured ectopic pregnancy may be associated with significant preoperative blood loss, but estimation of the extent of this is difficult because young women may have normal blood pressure despite a markedly reduced circulating blood volume. General anesthesia, with preparation for hemorrhage, is preferred if significant bleeding has occurred (e.g., ruptured tubal pregnancy) or is likely to occur (e.g., cervical, interstitial, cornual, cesarean scar, or abdominal ectopic pregnancy, or early placenta accreta). Intraoperative autologous blood transfusion can be used, and it may be useful especially in developing countries, where blood bank supplies are limited and women typically present late with significant hemoperitoneum and/or hypovolemic shock.37 The desire to preserve fertility often results in greater blood loss as tissue and organ preservation are attempted.
Abortion and Intrauterine Fetal Demise
Abortion refers to a pregnancy loss or termination, either before 20 weeks’ gestation or when the fetus weighs less than 500 g. It can occur spontaneously or may be performed electively for personal or medical reasons. A total of 825,564 elective abortions were reported in the United States in 2008, a rate of 16 abortions per 1000 women and a ratio of approximately 234 abortions per 1000 live births.38 The total number of abortions and rate of abortions (number of abortions per 1000 women) in the United States steadily declined from 1999 through 2007 and then remained static from 2007 to 2008.38 Between 1999 and 2008, the number, rate, and ratio (number per 1000 live births) of elective abortions in the United States declined by 3%, 4%, and 10%, respectively.38
In 2008, 62.8% of elective abortions were performed before 8 weeks’ gestation, 91.4% were performed before 13 weeks’ gestation, and 1.3% were performed after 21 weeks’ gestation.38 Most (75.9%) were performed by D and C at less than 13 weeks’ gestation, although some (14.6%) were induced medically, most commonly using methotrexate and misoprostol, or mifepristone and misoprostol before 8 weeks’ gestation.38
Of the 4693 reported pregnancy-related maternal deaths from 1998 to 2005 in the United States, 3% were the result of induced or spontaneous abortion.39 Deaths are usually the result of sepsis, hemorrhage, or embolism.40 Of the 20 abortion-related maternal deaths in the United States in 2003, 10 were related to spontaneous abortion and 10 women died after legal elective abortion (6 after surgical procedures and 4 after medical or nonsurgical procedures).
From a global perspective, abortion is a significant cause of maternal death. A review of causes of maternal death from 1997 to 2002 by the World Health Organization (WHO) reported that in some areas of Latin America and the Caribbean as many as 30% of maternal deaths are caused by abortion.41 In 2006, the WHO estimated that 12, 23, and 37 maternal deaths per 100,000 live births occur as a result of induced abortion in South Asia, Latin America/Caribbean, and sub-Saharan Africa, respectively.41 It is likely that many of these abortions are performed by unskilled individuals in a nonsterile environment that does not meet minimal medical standards. The exact number of maternal deaths that result from induced abortion is unknown and likely underreported.41,42
Spontaneous abortion occurs in 10% to 15% of clinically recognized pregnancies; when subclinical pregnancies are also considered, the incidence of spontaneous pregnancy loss may be as high as 60%.43 Although most spontaneous abortions manifest clinically at 8 to 14 weeks’ gestation, ultrasonography suggests that fetal demise usually occurs before 8 weeks’ gestation. If the fetus is viable at 8 weeks’ gestation, the incidence of subsequent fetal loss is only 3%.
The etiology of spontaneous abortion varies among patients. Chromosomal abnormalities are responsible for at least 50% to 80% of all spontaneous abortions.44 Other causes include (1) immunologic mechanisms, (2) maternal infections, (3) endocrine abnormalities (e.g., poorly-controlled diabetes mellitus), (4) uterine anomalies, (5) incompetent cervix, (6) debilitating maternal disease, (7) maternal clotting disorders, (8) trauma, and possibly (9) environmental exposures (e.g., irradiation, smoking, certain drugs).
Although some studies (conducted before scavenging of anesthetic gases was routine) suggested a higher incidence of spontaneous abortion among women who were exposed to trace concentrations of anesthetic agents in operating rooms,45 reevaluation of these data demonstrated significant flaws in study design, casting doubt on the original conclusions.46 Later studies have shown no increased incidence of spontaneous abortion in women working in operating rooms.47
Clinical Presentation and Obstetric Management
The clinical presentation and management of spontaneous abortion vary. A threatened abortion is defined as uterine bleeding without cervical dilation before 20 weeks’ gestation. Bleeding may be accompanied by cramping or backache. Once the diagnosis is confirmed, the patient’s activities are restricted until symptoms resolve. Approximately 25% of pregnancies are complicated by a threatened abortion; approximately half of affected women progress to a spontaneous abortion.48
An inevitable abortion is defined as cervical dilation or rupture of membranes without expulsion of the fetus or placenta. Spontaneous expulsion of the uterine contents usually occurs, but infection can be a complication.
A complete abortion
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


