Participants (# treated)
Trial design
Probiotic (strains)
Dosing (CFU/day)
Trial length (weeks)
Ref.
20 (10)
EBRPC
Blend Probiotic (Yakult™)
1 × 1010
12
[25]
102 (52)
DBRDD
Single strain (E. coli Nissle)
1 × 1011
12
[26]
90 (30)
R
Blend Probiotic (VSL#3™)
9 × 1011
8
[27]
16 (8)
DBRPC
Single strain + Prebiotic (B. longum)
4 × 1011
4
[28]
29 (14)
DBRPC
Blend Probiotic (VSL#3™)
1 × 1011/kg
52
[29]
120 (80)
R
Probiotic ± Prebiotic (B. longum)
2 × 109
4
[30]
90 (70)
DBRPC
Single strain (E. coli Nissle™)
1–4 × 109
2–8
[31]
147 (77)
DBRPC
Blend Probiotic (VSL#3™)
7.2 × 1012
12
[32]
144 (71)
DBRPC
Blend Probiotic (VSL#3™)
3.6 × 1012
8
[33]
For the three included studies:
probiotics (Yakult™) + 5-ASA had similar effectiveness (40 %) to placebo + 5-ASA (30 %) for induction of remission [25]: OR 0.64 (95 % CI: 0.10–4.10);
probiotics (E. coli Nissle 1917) + steroids had similar effectiveness (68.4 %) as mesalazine + steroids (74.6 %) for induction of remission [26]: OR 1.35 (95 % CI: 0.6–3.04).
probiotics (VSL#3™) + balsalazide had similar effectiveness (80 %) to placebo + balsalazide (70 %) for induction of remission [27]: OR 0.58 (95 % CI: 0.18–1.91).
As comparison, in a trial of 268 UC patients with moderate severity 4.8 g of delayed release oral mesalamine was found to have clinical benefit in 70 % and superior to a response rate of 59 % for those using a lower dose of 2.4 g of a delayed release oral mesalamine for moderate UC disease activity [28].
There have been some additional randomized placebo-controlled trials studies since the Cochrane systematic review (Table 29.1, refs. [30–34]). Two of these more recent studies [31,34] were also included in another meta-analysis that also failed to find remission rates significantly different with total remission rate of probiotics auxiliary therapy at 68.2 % and nonprobiotics groups 60.4 % (OR 1.35; 95 % CI: 0.98–1.85, p = 0.07).
The Miele et al. [34] trial recruited children with moderate-to-severe disease, VSL#3™ or placebo was administered along with corticosteroids and mesalamine. The corticosteroid dose (1 mg/kg/day to a maximum of 40 mg/day) and mesalamine (50 mg/kg/day) dose were those commonly used. The corticosteroids were tapered after a month if subjects were in remission. In this study, remission was achieved in 13 of 14 participants (92.8 %) treated with VSL#3™ and IBD therapy and in 4 of 15 patients (36.4 %) treated with placebo and IBD therapy (p < 0.001). This result must be taken in context the response rate to corticosteroids and mesalamine in the placebo-treated group. As a comparison, in a multicenter North American registry reporting the outcome of children with newly diagnosed UC, 60 % of those treated with corticosteroids were in remission at 3 months [35].
Using a quality of life measure Fujimori and colleagues [30] studied patients on stable doses of aminosalicylates and/or prednisolone for at least 4 weeks in remission or had mildly active UC. The probiotic was taken once daily and the prebiotic twice daily, and the study was not double-dummy controlled. The only measure of disease activity was C-reactive protein (CRP) on a small number from each group. Only those patients taking a combination of a prebiotic and Bifidobacterium longum had an improvement (p = 0.03) whereas those subjects on the individual components (either prebiotic alone or probiotic individually) did not.
In a multicenter, randomized, double-blind, placebo-controlled trial from India, Sood et al. [31] studied the blend probiotic VSL#3™ in adults with mild-to-moderate UC. Participants were assigned randomly to groups that were given 3.6 × 1012 CFU VSL#3 (n = 77) or placebo (n = 70) twice daily for 12 weeks. A primary endpoint of 50 % decrease in the Ulcerative Colitis Disease Activity Index (UCDAI) at 6 weeks was achieved in a greater number of those receiving probiotic (32.5 %) than the group given placebo (10 %) (p = 0.001). A secondary endpoint of remission by 12 weeks was achieved in 33 subjects given probiotic (42.9 %) compared with 11 subjects given placebo (15.7 %, p = 0.001).
The response to rectal enemas of E. coli Nissle in subjects with distal proctitis of moderate activity was studied by Matthes et al. [32]. The concentration of the probiotic was 108 CFU/mL and subjects with mild-to-moderate disease activity were randomized to receive enemas once daily containing either 10 mL, 20 mL, 40 mL, or placebo that was volume-matched the three different enema volumes used in the E. coli Nissle groups. Permissible concomitant therapies included loperamide drops to improve retention capacity for enemas, and oral UC maintenance treatment with aminosalicylates or steroids at a constant level for at least 2 weeks prior to the study. A disease activity index was used to measure response and if there was no response at 2 weeks, they were classified nonresponders but otherwise could continue up to 8 weeks of therapy. In contrast to per protocol analyses, intention-to-treat analysis revealed the number of responders was not significantly higher in the E. coli Nissle group than in the placebo group (p = 0.4430) in this Phase II study.
Tursi and colleagues [33] also evaluated VSL#3 in adults with relapsing mild–moderate UC as additional therapy to their usual 5-aminosalicylate and/or immunomodulator therapy. A total of 3.6 × 109 CFU/day (two sachets twice a day) of the blend of probiotics were ingested for the 8-week study period. The primary outcome was a 50 % decrease in the Ulcerative Colitis Disease Activity Index newly described in the manuscript that includes weighted parameters for stool frequency, amount of rectal bleeding, mucosal appearance and physician’s rating for disease activity. There is no information with regards to the validation of this scale and it is significantly different from the validated Pediatric Ulcerative Disease Activity Index [36]. A greater percentage of those receiving the probiotics achieved the primary outcome (63.1 vs. 40.8; Intention to treat p = 0.031, 95 % CI: 0.47–0.69). However, secondary outcomes of stool frequency, physician’s assessment of disease activity, and endoscopic scores were similar between the two groups.
Maintenance of Remission
Naidoo and colleagues performed a Cochrane review on probiotics for maintenance of remission in ulcerative colitis [37]. There were four studies included and those excluded were those that subjects were followed for less than 3 months, patients were not in remission at the start of the study or not a randomized controlled trial. As detailed in Table 29.2, the total number of subjects receiving probiotic treatment is 409 among these four studies with three different probiotic preparations being used along with differing doses of probiotics being administered. A relapse rate was similar between those receiving probiotics compared to three studies that controls received mesalamine (n = 555; 40.1 % vs. 34.1 %; OR 1.33; 95 % CI: 0.94–1.90). In the Wildt study [41], there was no difference in the relapse rate at 1 year comparing the blend probiotic preparation (Lactobacillus acidophilus LA-5 + Bifidobacterium animalis BB-12) with placebo (n = 32; 75 % vs. 92 %; OR 0.27; 95 % CI: 0.03–2.68). Given the number of patients studied, study bias and event number, the authors concluded there was insufficient evidence to make conclusions about efficacy of probiotics for maintenance in UC.
Table 29.2
Randomized controlled trials of probiotics used as maintenance therapy for at a minimum of 3 months for ulcerative colitis
Participants (# treated) | Trial design | Probiotic (strains) | Dosing (CFU/day) | Trial length (Months) | Ref. |
|---|---|---|---|---|---|
103 (50) | DBRDD | Single strain (E. coli Nissle) | 5 × 1010 | 3 | [38] |
327 (162) | DBRDD | Single strain (E. coli Nissle) | 5 × 109 | 12 | [39] |
187 (127) | R | Single strain (L. rhamnosus GG) | 1.8 × 1010 | 12 | [40] |
32 (20) | DBRPC | Blend (L. acidophilus LA-5, B. animalis BB-12) | 1.5 × 1011 | 13 | [41] |
Pouchitis
Pouchitis is the most common complication of the surgery and although the exact etiology is not clear host genetic factors, local pouch issues and the microbiota contained within the pouch, are thought to be involved (see chapter on Pouchitis). Most patients will develop this problem in the first year and antibiotics can be an effective form of therapy in many [42,43]. Although some patients are antibiotic-resistant and others improve on antibiotics, but there is a relapsing course of their pouchitis following the discontinuation of antibiotics. As antibiotics can provide relief for most with pouchitis, a basic assumption has been the importance of the microbiota of the pouch in the development and chronicity of pouchitis. Therefore alteration/modulation of their pouch reservoir microbiota by addition of probiotics for the treatment of acute pouchitis, prevention of initial onset of pouchitis, and prevention of relapsing pouchitis have all been evaluated.
Probiotics as Treatment of Pouchitis
Trials for treating mild/moderate pouchitis are few with small numbers of adult participants. Kuisma et al. [44] recruited 20 patients (ten intervention arm) for a DBRPC trial of Lactobacillus rhamnosus GG 2 × 1010 CFU/day for 3 months. Those patients with chronic, active pouchitis were excluded. The Pouchitis Disease Activity Index [45] was utilized for evaluation of clinical effect and prior to study entry, the mean PDAI was in the mild range (8.0 ± 0.8). There was no difference following the intervention period with clinical response (defined as a PDAI score reduction of ≥3) occurring in one of ten (10 %) patients in the probiotic group and zero of ten (0 %) patients in the placebo group (10 % vs. 0 %, p = 0.32).
In an open-label trial of 51 UC patients postileal pouch-anal anastomosis using a fermented milk product with a blend of probiotic strains (L. acidophilus strain La5 + Bifidobacterium lactis strain Bb12) containing 5 × 1010 CFU/day [46] however, there was a reported improvement in endoscopic evaluation. In another open-label trial, 23 consecutive patients with mild pouchitis as defined using Pouchitis Disease Activity Index (scores 7–12) were treated with 3.6 × 1012 CFU/day of VSL#3™ for 4 weeks [47]. Sixteen of 23 patients (69 %) with mild pouchitis were in remission after treatment and the median total Pouchitis Disease Activity Index scores reported before therapy improved following therapy (ten vs. four, p < 0.01).
Thus, there is limited evidence for a role of probiotics as monotherapy for mild/moderate pouchitis at the present time. Limiting access of microbiota to the mucosa of the pouch and subsequent development of inflammation may be a mechanism whereby probiotics provide benefit. Alternatively, changing the composition of the pouch microbiota may be important; although it is interesting that no long-term colonization of probiotic strains is achieved [48]. Thus it may not be surprising that once the deleterious microbiota have colonized within the pouch there is little a probiotic as monotherapy can do to alter the situation. A somewhat analogous situation exists for use of probiotics as monotherapy in treating Helicobacter pylori gastritis. The eradication rate of probiotic monotherapy was poor compared to standard triple therapy (a proton pump inhibitor + two antibiotics) in children colonized with H. pylori [49] but a number of studies have reported reduced H. pylori colonization with probiotic monotherapy even though eradication rate is poor [50]. In addition, one study suggested reduced gastritis on biopsy [51]. The increased eradication rates of H. pylori using combined probiotic and antibiotic may take advantage of lower levels of pathogen in the stomach and/or decreased adverse effects of the antibiotics. Thus, if there is an analogy to be drawn for it would be to treat patients with pouchitis requiring continuous antibiotics or very frequent use of antibiotics with probiotics as adjuvant to shorter antibiotic courses of therapy.
Prevention of Initial Postoperative Onset of Pouchitis
Two trials have studied whether there is an advantage to initiate probiotics immediately following ileal pouch-anal anastomosis and both found there to be benefit to the delay in onset of development of pouchitis. In a placebo-controlled trial [52] at the end of 1 year (see Table 29.3), only 2 of 20 (10 %) of those in the intervention probiotic arm had developed colitis as compared to 8 of 20 (40 %; no episodes 80 % vs. 60 %, p = 0.03) of the control arm participants as determined using the PDAI with endoscopy. The Peto odds ratio for prevention of pouchitis by the probiotic (VSL#3™) compared with placebo was 4.76, 95 % CI: 1.16–19.56.
Table 29.3
Randomized trials of probiotics in prevention of onset or recurrence of pouchitis
Participants (# treated) | Trial design | Probiotic (strains) | Dosing (CFU/day) | Trial length (Months) | Ref. |
|---|---|---|---|---|---|
40 (20) | DBRPC | Blend (VSL™) | 9 × 1011 | 12 | [52] |
40 (20) | DBRPC | Blend (VSL™) | 1.8 × 1012 | 9 | [53] |
36 (20) | DBRPC | Blend (VSL™) | 9 × 1011 | 12 | [48] |
Maintenance of Pouchitis Remission
The initial controlled trial for this indication was in the year 2000 using the blend probiotic product, VSL#3™ and reported an outstanding effect in prevention of the recurrence of pouchitis in patients with antibiotic-dependent pouchitis. Prior to the administration of the blend probiotic, participants in his trial were successfully treated with a combination of antibiotics (ciprofloxacin + rifaximin). At the end of the study period of 9 months, only 3 of 20 (15 %) had developed pouchitis in the probiotic intervention group whereas all 20 participants in the control group had a recurrence of pouchitis and occurred by 4 months following antibiotics cessation [53]. A similar result was noted in another European trial of VSL#3™ (see Table 29.3) also evaluating prevention of recurrence of pouchitis in relapsing or chronic pouchitis patients [48]. Remission of the pouchitis was induced in these participants by administering 4 weeks of a combination of antibiotics (metronidazole + ciprofloxacin) that was followed by either VSL#3™ or a placebo to then evaluate the preventative effects of the probiotics. In the probiotic treatment group remission was maintained in 17 of 20 (85 %) but only 1 of 16 (6 %, p < 0.0001) on placebo. The pooled Peto odds ratio for these two studies for the combined rate of maintenance of remission with probiotic bacteria compared to placebo (97 % vs. 3 %, p < 0.0001) was 25.39 (95 % CI: 10.37–62.17). The number needed to treat with oral probiotic therapy to prevent one additional relapse was 2 [54].
In contrast, an open-label trial by Shen and colleagues [55] reported less responses in prevention of pouchitis recurrence using the same probiotic. In their trial, 31 subjects were prescribed a 2-week treatment of a single antibiotic (ciprofloxacin) followed by VSL#3™. Also in contrast to the other studies, the VSL#3™ was bought by patients rather than be supplied through the study. Probiotic therapy was stopped by 9 of 31 (29 %) 7 weeks into therapy and 25 of 31 (81 %) by 8 months had discontinued the probiotic because of failure to prevent pouchitis (n = 23) or side effects of the probiotic administration (n = 2). Only 6 of 31 (19 %) did not develop clinical evidence of pouchitis by the end of the 8-month trial period. Even among these six subjects, endoscopy revealed some level of pouch inflammation. In this trial [55], there was a single antibiotic administered and endoscopy was not performed prior to probiotic administration to ensure pouch inflammation had completely resolved.
Out of all of this is a recent clinical practice guideline on management of pouchitis [43] suggesting that for those patients with prompt recurrence of pouchitis following antibiotic usage or having multiple recurrences of pouchitis despite antibiotics either VSL#3™ or chronic use of antibiotics is a strategy to consider. Probiotics alone were not recommended for acute treatment of pouchitis.
Crohn Disease
Induction of Remission
There is a paucity of studies investigating use of probiotics to settle active inflammation. In two open-label studies [56,57], probiotics (using combination of Bifidobacterium breve + Lactobacillus casei + B. longum + prebiotics or L. rhamnosus GG, respectively) were added as adjuvant therapy to immunomodulators and corticosteroids. In the former study [56], seven of ten patients were reported to respond as determined by Crohn Disease Activity Index scores with most noticeable improvement in the diarrhea. However, there was no improvement in inflammation as measured by erythrocyte sedimentation rate (ESR) and CRP. In an open-label trial using L. rhamnosus GG [57], three of the four children were reported to have improved Pediatric Crohn Disease Index (PCDAI) scores or serial determinations over the 6 months of the trial. Specifics with regards to ESR or CRP are not reported although the ESR is a component of the PCDAI [58].
A placebo-controlled trial using L. rhamnosus GG was the sole study included in a Cochrane review of efficacy of probiotic supplementation for the induction of remission in CD [59]. There were a total of 11 participants in the study and subjects received antibiotics and concurrent therapy of corticosteroids and some methodological concerns were raised. Four of five patients in the probiotic group achieved remission compared to five of six in the placebo group (OR 0.80; 95 % CI: 0.04–17.20). Thus, this one small study did not show that probiotics had any effect in treating active Crohn disease. At best, one could say there is insufficient evidence to make any conclusions about the effectiveness of probiotics for treatment of active Crohn disease.
Maintenance of Remission
Initial randomized trials (see Table 29.4) in Crohn disease were reported with probiotics used as sole maintenance therapy following corticosteroid therapy or in combination with 5-aminosalicylate therapy for maintenance therapy in those already in remission as adjuvant therapy [60–63]. Among these studies [60–62], there were no differences in the number of relapses in patients receiving E. coli Nissle compared to the placebo (p = 0.11), S. boulardii (1 g/day) plus mesalazine (2 g/day) compared to mesalazine alone (3 g/day) (p = 0.08), or patients with remission induced medically and then receiving L. rhamnosus GG (p = 0.77).
Table 29.4
Randomized trials of probiotics for maintenance of remission of Crohn disease
Participants (# Treated) | Trial Design | Probiotic (Strains) | Dosing (CFU/day) | Trial Length (Months) | Ref. |
|---|---|---|---|---|---|
28 (16) | DBRPC | Single strain (E. coli Nissle 1917) | 5 × 1010 | 12 | [60] |
32 (16) | R | Single strain (S. boulardii) | N/A | 6 | [61] |
11 (5) | DBRPC | Single strain (L. rhamnosus strain GG) | 2 × 109 | 6 | [62] |
75 (39) | DBRPC | Single strain (L. rhamnosus strain GG) | 2 × 1010 | 24 | [63] |
In the largest maintenance trial to date, Bousvaros et al. [63] also reported no difference in relapse rate for subjects receiving L. rhamnosus strain GG 2 × 1010 CFU/day (31 %; 12 of 39) or placebo (17 %; 6 of 36, p = 0.18). The time to relapse is shown in Figure 29.1, and although there was a trend in those receiving probiotics to a shorter time to relapse, comparison between it and the placebo control group was not statistically different (p = 0.10) in this study.
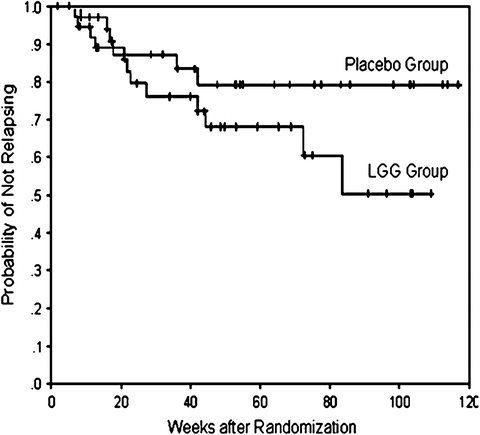

Fig. 29.1




Kaplan–Meier survival curve showing the probability of staying relapse-free during the duration of the study treatment duration for participants administered L. rhamnosus GG or placebo. Source: Figure reproduced from Inflamm Bowel Dis 2005;11:833–839. Copyright © 2005 Crohn’s & Colitis Foundation of America, Inc.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


