CHAPTER 36 Principles of radiotherapy and chemotherapy
Radiotherapy
Radiation physics
Electromagnetic radiation
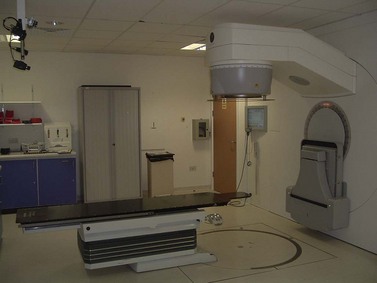
X-rays and gamma-rays (γ-rays) are part of the electromagnetic spectrum; the former are produced artificially and the latter from naturally occurring or artificially produced radioisotopes. Megavoltage X-rays for therapeutic purposes are produced by a linear accelerator (Figure 36.1) by accelerating electrons to high kinetic energies on to a target composed of tungsten or gold. Kinetic energy given up by the electrons is converted to high-energy X-rays. γ-rays are emitted as byproducts of radioactive isotopes (e.g. iridium-192) undergoing decay to reach a stable nuclide.
Radiobiology
External beam radiotherapy
Conformal radiotherapy
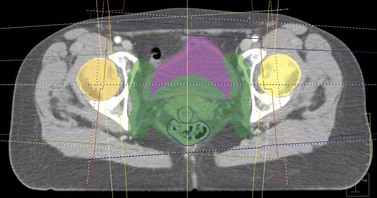
The CT scan images are subsequently transferred to a computer terminal (Figure 36.2) with software allowing delineation of target volumes and organs at risk according to recommendations from the International Commission on Radiation Units and Measurements. The gross tumour volume includes the palpable and/or radiologically visible tumour; it is absent if it has been surgically resected. The clinical target volume (CTV) involves the gross tumour volume and potential areas of microscopic disease (e.g. pelvic lymph nodes and parametria in cervical cancer). The CTV is grown by a margin to allow for internal organ motion and geometric uncertainties in treatment delivery; this is called the ‘planning target volume’ (PTV). The organs at risk for pelvic radiation include the rectum, bladder, small bowel and both hip joints. The kidneys and spinal cord are the dose-limiting organs at risk when radiating the para-aortic lymph nodes. The clinician specifies the dose fractionation to be used in the radiation treatment of the patient.
The final radiotherapy plan comprises the following information: the number of beams used to optimize conformity to the PTV, beam energies, beam direction, use of wedge angles and multi-leaf collimators to improve PTV conformity, and the dose distribution and percentage dose received by the PTV. A dose–volume histogram is provided to determine the dose being received by the PTV and organs at risk. The final plan is checked and approved by the clinician prior to implementation of the radiotherapy plan (Figure 36.3). Verification checks are performed to validate the treatment plan and to detect for any unforeseen errors prior to starting radiation treatment. A standard dose prescription for carcinoma of the cervix is 45–50 Gy in 25–28 fractions using 1.8 Gy/fraction over 5–5.5 weeks.
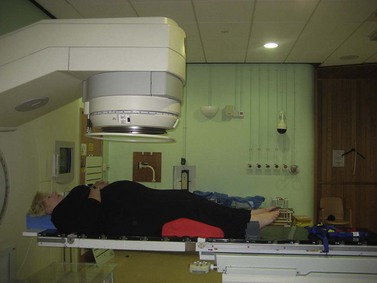
The patient is treated in a specially designed room to prevent radiation of personnel outside the room. The patient is positioned on the treatment couch with reference to the tattoo marks (Figure 36.4). Computerized transfer of planning data from the planning computer to the linear accelerator minimizes the risk of human error in transferring data. The radiographers control the linear accelerator from outside the room using a console which is used to start and stop the radiation beam, and to set the duration of treatment and the dose to be delivered. The patient is monitored using cameras in the treatment room, and communication with the patient is possible via a microphone. These safety features are designed to optimize radiation treatment and are subject to frequent quality assurance checks.
Virtual simulation
The planning CT scan images can be reconstructed using computer software to provide a digitally reconstructed radiograph (DRR). Bony landmarks are used for radiotherapy planning with the clinician using the computer software to place the radiation beams on to the DRR. This is akin to conventional radiotherapy. The advantages of virtually simulated radiotherapy planning include: the clinician is able to visualize the pelvic soft tissues by using the CT scan images, thus ensuring the tumour is encompassed within the radiation field; radiotherapy planning is less time-consuming compared with conformal radiotherapy; the clinician can apply multi-leaf collimators at the time of planning to optimize shielding of the organs at risk; and a dose distribution can be provided by the medical physicists to ensure that the PTV is receiving the prescribed dose. The disadvantages of virtually simulated radiotherapy planning are that it is not suitable for producing complex radiotherapy plans, and a dose–volume histogram cannot be provided to determine the dose being received by the organs at risk. Virtually simulated radiotherapy (Figure 36.5) is frequently utilized for pelvic radiotherapy for the reasons outlined above.
Conventional radiotherapy
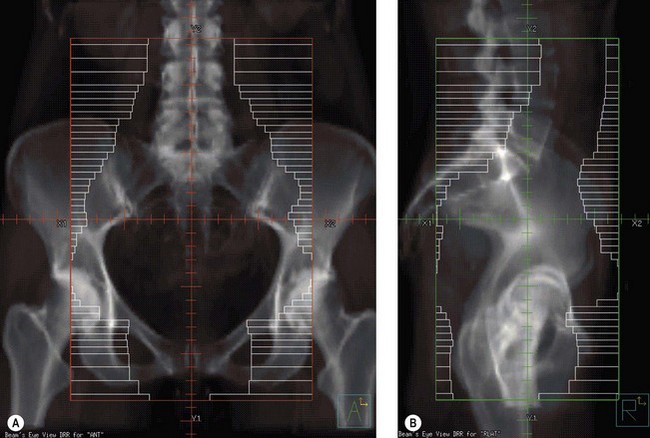
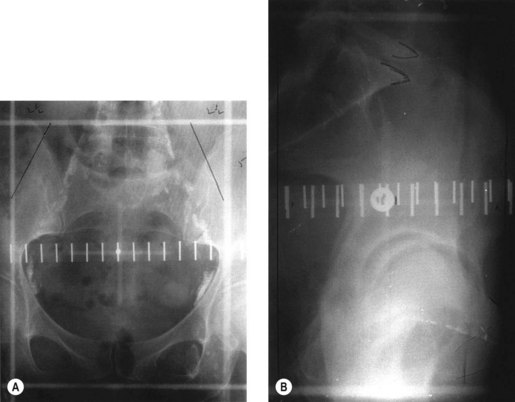
Conventional external beam radiotherapy uses bony landmarks to define the target volume for pelvic radiotherapy. A simulator film is obtained after the patient is aligned on the simulator couch using orthogonal lasers. Cross wires mounted in the light beam from the simulator define the size of the area to be treated, and anterior–posterior and lateral pelvic X-rays are obtained as permanent records. The clinician is able to identify areas where shielding can be applied to reduce dose to the organs at risk (Figure 36.6). As diagnostic X-rays have poor soft tissue resolution, the clinician must rely on information gathered from clinical examination and radiological investigations to determine the target volume. Studies have reported underdosing of the regional lymph nodes in up to 40% of patients using conventional radiotherapy. However, conventional radiotherapy planning still has a role in centres where access to CT planning software is restricted due to resource implications, in patients unable to fit into a CT scan machine due to gross obesity, and in palliative radiotherapy.