Breast milk transmission of infections (e.g. CMV, GBS, HIV) is considered in Chapter 19.
23.1.2 Early enteral feeding
Early enteral feeding could reduce late-onset neonatal infections by reducing the need for central venous access and parenteral nutrition, both known risk factors for late-onset sepsis (see Section 2.2.4).
Theoretical suggestions that early enteral feeds might predispose to NEC have been refuted by trial-based evidence (see Chapter 11).17–19 A Cochrane systematic review of nine trials (754 infants) comparing early trophic feeding versus fasting for infants <;1500 g found no difference in the incidence of NEC.17 Only two trials reported rates of sepsis. One RCT compared minimal enteral nutrition from days 2 to 7 with fasting, but feeds were increased at the same rate from day 8 and not surprisingly there was no difference in incidence of sepsis.20 Another RCT randomized infants <;1750 g to trophic feeds or (0.5–1 mL/hour plus parenteral nutrition from day 3 until ventilator support finished) or parenteral nutrition alone.14 Trophic feeding infants had significantly fewer infections (mean 0.5 vs. 1.2 episodes of culture-proven sepsis), received less parenteral nutrition (mean difference 11.5 days), grew better and were discharged home earlier.14 The additional infections in the parenteral nutrition group were mainly due to catheter-associated organisms (CoNS and to a lesser extent S. aureus).14
In a retrospective study from Israel of infants <;1500 g, 42% developed nosocomial infection and 9% NEC.21 Enteral feeding was introduced at the same age (3–4 days) in infants who did and did not develop NEC. However, enteral feeding was introduced significantly later (4.8 vs. 2.8 days) in infants who developed late-onset infection. The mean age at start of feeding fell consistently over the 6-year study period, correlating with a concomitant fall in late-onset infections. This study is susceptible to confounding: the fall in infections with time could be due to many other factors.21
Although more RCTs would be welcome, the evidence from one well-conducted RCT14 and one observational study21 supports early trophic feeds for very low birth-weight infants as being beneficial and not harmful (Table 23.1).
23.1.3 Probiotics
Probiotics, naturally occurring gut bacteria such as Lactobacillus and Bifidobacterium found in the stools of full-term breastfed infants, can be given as live microbial supplements to colonize the gastrointestinal tract.
A Cochrane systematic review found 16 randomized or quasi-randomized trials of probiotics in 2842 pre-term infants.15 Probiotics were associated with significant reductions in severe NEC (typical relative risk (RR) 0.35, 95% CI 0.24–0.52) and in mortality (typical RR 0.40, 95% CI 0.27–0.60). There was no reduction in nosocomial infections (typical RR 0.90, 95% CI 0.76–1.07).15 A non-Cochrane systematic review found similar reductions in NEC and mortality with no difference in rates of blood culture-proven late-onset infection.16 Neither review reported sepsis caused by probiotic organisms, one theoretical concern that has been raised.
These findings have provoked controversy (see Chapter 11). The largest studies were in Italy, Taiwan and Turkey and some critics have questioned their applicability to other settings. Different probiotic formulations were used and in some countries probiotics are not licensed for neonatal use. At the time of writing, results are awaited from large RCTs in Western countries which will provide more evidence (Table 23.1).
Probiotics do not reduce mortality by reducing sepsis or NEC. Interestingly, however, prolonged antibiotics are associated with increased all-cause mortality and NEC in very low birth-weight infants.22,23 It is tempting to postulate that probiotics act by replacing protective gut micro-organisms eradicated by broad-spectrum antibiotics.
23.1.4 Prebiotics
Prebiotics are non-digestible oligosaccharide food components that selectively stimulate the growth or activity of non-pathogenic bacteria in the colon.24 In a systematic review and meta-analysis of four RCTs in 126 pre-term infants prebiotic supplemented formula increased stool colony counts of probiotic organisms (bifidobacteria and lactobacilli), but there was no effect on the rates of infection.25 A systematic review of six RCTs in 1459 full-term infants found that prebiotics did not affect neonatal infections.26 (Table 23.1).
23.2 Infection control measures
23.2.1 Hand washing
Hand hygiene (hand washing) is compulsory in the computer microchip industry, because microchips can be permanently damaged if handled with unwashed hands, and non-compliant workers can be disciplined or even dismissed.27 Hospitals are busy and stressful and hand washing lapses when health-care workers’ workload increases.28 Nevertheless, it is not easy to explain to parents that health-care workers forget to wash their hands when they are busy. It is shameful that microchips are valued higher than babies.29
Does hand hygiene reduce nosocomial infections?
Studies of improved hand hygiene and nosocomial neonatal infections are generally before-and-after intervention studies which often do not measure compliance (Table 23.2). A systematic review of hand hygiene and nosocomial infections found only 35 studies that met the inclusion criteria and concluded ‘the varied nature of the interventions used and the diverse factors affecting the acquisition of health-care associated infections make it difficult to show the specific effect of hand hygiene alone’.41 In Western countries most of the reduction is in CoNS infections,30–32 although before-and-after studies suggest improved hand hygiene also reduces spread of virulent or multi-resistant colonizing pathogens 33,34 and respiratory virus infections.35
Table 23.2 Infection control interventions to prevent neonatal infection.
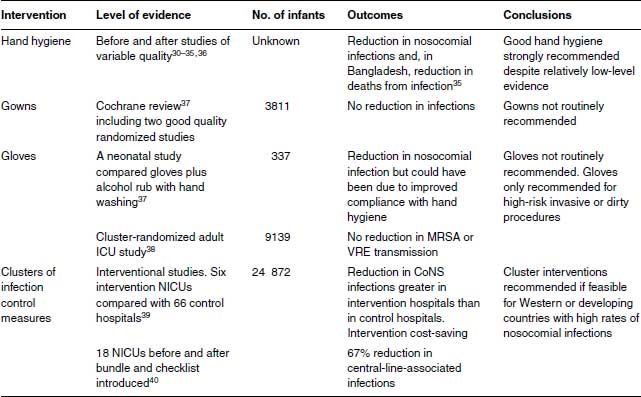
In developing countries, there are few well-conducted studies. Introduction of an infection control programme in Bangladesh emphasizing improved hand washing was followed by a 61% decrease in culture-proven sepsis and in deaths from clinical (82%) or culture-proven sepsis (50%).42
How well do we wash our hands?
A non-Cochrane systematic review found 96 empiric studies on compliance with hand hygiene, 65 in intensive care units.43 The overall median compliance rate was an embarrassing 40% and rates were lower in intensive care units (30–40%) than elsewhere, presumably due to workload. Doctors (32%) were worse than nurses (48%).43
Does it matter what we use for hand hygiene?
Alcohol rubs remove more bacteria from hands than soap and water,44 but there is little evidence that this equates with fewer nosocomial infections. A cross-over study in two US neonatal units compared a traditional antiseptic hand-wash with an alcohol hand sanitizer. Infection rates and microbial counts on nurses’ hands were no different, although nurses’ skin improved when using alcohol.45 In an Italian study, nosocomial infections were reduced when a standardized hand hygiene programme was introduced using chlorhexidine antimicrobial soap and alcohol-based hand rubs compared with plain detergent (triclosan) but the study did not examine compliance.46 Soap and water were more effective than alcohol rubs in reducing office workers’ infections in a cluster-randomized study in Finland,47 but were no different when compared in US elementary schoolchildren.48 A large cluster-randomized surgical study in Kenya found no difference in surgical site infections irrespective of whether alcohol-based rub or plain soap and water was used for surgical hand preparation.36 Alcohol-based hand rubs are cheap and a good alternative when continuous clean water is unavailable.36
How can we improve hand hygiene compliance?
Medical and nursing staff and other health-care workers are well aware that hand washing is important, but compliance is a major issue. A Cochrane systematic review found only four studies of hand hygiene compliance describing different interventions with inconclusive results.37 In a non-Cochrane systematic review, low compliance was associated with high activity levels and with physician involvement.43 Compliance was higher in association with dirty tasks, availability of alcohol-based hand rub or gel, improved accessibility of hand hygiene materials and with performance feedback.43
An Australian children’s hospital reported a sustained increase in hand hygiene compliance from 23% in 2006 to 87% in 2011 following a ‘Clean hands save lives’ campaign involving education, improving alcohol-based hand rub accessibility at points of patient care, monitoring staff practices and feeding back performance data.37 This was associated with a decline in nosocomial bacteraemia and in transmission of multi-resistant organisms.49
23.2.2 Gowns
A Cochrane systematic review of gowning by attendants and visitors in neonatal units found that only two of eight studies were of good quality. Not wearing gowns was associated with a trend to lower death rate (typical RR 0.84, 95% CI 0.70–1.02). Gowning had no effect on nosocomial infections (typical RR 1.24, 95% CI 0.90–1.71).50 Routine gowning is not recommended (Table 23.2).
23.2.3 Gloves
There is no evidence that gloves have any advantage over hand hygiene. A Hong Kong sequential study compared 36 months of conventional hand washing with 36 months of alcohol hand rub used by workers wearing non-sterile gloves.51 Nosocomial infections fell but compliance was not assessed. Universal gloving did not reduce transmission of MRSA or VRE compared with standard infection control practices in a large cluster-randomized trial in US adult intensive care units.38
Routine use of gloves is expensive and unpopular with staff. Gloving is obviously important for high-risk invasive procedures such as central venous catheter insertion and to protect staff with broken skin on their hands, for example, eczema, against infections (Table 23.2).
23.2.4 Clusters of infection control interventions
Clusters of different infection control interventions introduced simultaneously have become popular in adult and paediatric intensive care units. Sequential studies or preferably cluster-randomized trials can be used to evaluate efficacy of the whole cluster. This does not generate evidence on individual components which may not matter if the cluster is effective and cost-effective. The Vermont Oxford Network leads the way in this field in neonatal infection prevention, initially in Western countries and subsequently in resource-poor settings.39,52
In a sequential before-and-after study late-onset CoNS infections fell from 22% to 16.6% of infants <;1500 g in US neonatal units assigned to a 3-year collaborative quality improvement intervention.39 Nosocomial infections with other organisms did not fall.39 The intervention reduced the costs of neonatal care while the costs rose in control hospitals and the intervention was assessed as cost-effective.52
An innovative study compared 15 differences in infection control practices in US NICUs with high and low rates of nosocomial infections, shared them with all the hospitals and let individual NICUs decide which practice differences to implement.53 The network nosocomial infection rate fell from 3.8 to 2.9 episodes per 1000 patient-days.53
A statewide intervention in New York State aimed to reduce central-line-associated bacteraemias (CLABs). All 18 NICUs adopted central-line insertion and maintenance bundles and checklists to monitor their use. The before and after study found a 67% reduction in rates of CLAB (95% CI 59–73).40
A Canadian cluster-randomized study assigned six NICUs to a cluster of evidence-based interventions to reduce nosocomial infections (strategic placement of hand cleaner dispensers, measures to reduce skin breaks and measures to reduce duration of antibiotics) and six NICUs to interventions to reduce bronchopulmonary dysplasia (BPD).54,55 There was a significant reduction in BPD but a non-significant reduction in nosocomial infections. Cost-effectiveness was not assessed.55
23.3 Physical interventions
23.3.1 Umbilical cord care
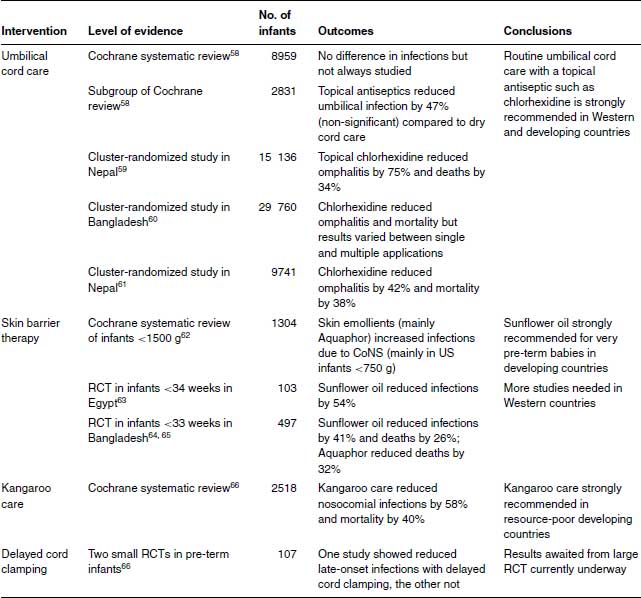
Historically, improved umbilical cord care was associated with dramatic reductions in omphalitis and in skin (impetigo) and systemic infections (osteomyelitis, disseminated sepsis) with Staphylococcus aureus.56,57 A Cochrane systematic review of umbilical cord care found 21 studies (8959 participants) mainly from high-income countries.58 Antiseptics reduced colonization with S. aureus significantly by about half. Umbilical infection was rare (about 1%) and not reported in most studies. Antiseptic cord care compared with dry cord care was associated with a non-significant but almost 50% reduction in umbilical infection (9 of 1431 vs. 18 of 1400, RR 0.53, 95% CI 0.25, 1.13).58
Evidence of the importance of cord care is much stronger in developing countries, particularly three recent cluster-randomized trials. A community-based study in Nepal randomized over 15 000 infants to cord care with 4% chlorhexidine, soap and water or dry cord care.59 Topical chlorhexidine reduced severe omphalitis by 75% (95% CI 47–88) compared to dry cord care and reduced mortality by 34% (95% CI 5–54) for infants enrolled within 24 hours of birth.59 In Bangladesh, 29 760 babies were randomized to single cleansing with chlorhexidine as soon as possible after birth, multiple cleansing daily chlorhexidine for 7 days or dry cord care.60 Neonatal mortality was 20% lower in the single-cleansing group than dry cord care. Severe omphalitis was reduced in the multiple-cleansing but not the single-cleansing group compared to dry cord care. A Pakistan study randomized 9741 babies to combinations of chlorhexidine or promotion of hand washing in the family. Hand-washing promotion was ineffective, but chlorhexidine was associated with a 42% reduction in omphalitis (95% CI 18–59) and a 38% reduction in mortality (95% CI 15–55).61
There were no safety concerns with the use of chlorhexidine which is strongly recommended in developing countries (Table 23.3).
Table 23.3 Physical interventions to prevent neonatal infection.
23.3.2 Skin barrier therapy
Skin barrier therapy using emollient ointments may reduce heat loss and skin breakdown in very pre-term babies and possibly nosocomial infections. A Cochrane systematic review62 of four RCTs (1304 infants) reported borderline increased incidence of any nosocomial infection in babies who received skin barrier therapy (RR 1.20, 95% CI 1–1.43), almost entirely due to CoNS infections in babies 501–750 g (RR 1.31, 95% CI 1.02–1.70). However, 92% of the babies in the Cochrane review came from a single US study performed by the Cochrane authors, using a proprietary product, Aquaphor, comprising petrolatum, mineral oil, mineral wax and lanolin alcohol.
Studies in developing countries led by Gary Darmstadt and colleagues from Johns Hopkins University, Baltimore show sunflower seed oil is cheap and highly effective at reducing infections and deaths in pre-term babies.63–65 An Egyptian study of infants <;34 weeks of gestation found that 8 hourly topical sunflower oil was associated with a 46% reduction (95% CI 19–74) in nosocomial infections.64 The cost was about US $0.20 a course. An RCT of infants <;33 weeks in Bangladesh compared sunflower oil or Aquaphor with untreated controls.65,67 Either sunflower oil or Aquaphor reduced nosocomial infections by 40%, although only the former result reached significance.64 When the study was continued, they both reduced mortality significantly: sunflower oil by 26% (95% CI 1–45) and Aquaphor by 34% (95% CI 8–49).65,67
The number needed to treat (NNT) is three or four babies. The total cost is less than US $1 to save a life. This is arguably the most cost-effective intervention in neonatal infection prevention.
23.3.3 Kangaroo care
Kangaroo care or kangaroo mother care was developed by Edgar Rey in 1978 in Bogota, Colombia, as an alternative to conventional methods of caring for low birth-weight infants, because of a local lack of incubators and a high rate of nosocomial infections.68 The name refers to using skin-to-skin contact between a mother and her newborn to provide warmth and comfort: babies are placed between the mother’s breasts and held in place with a wrap (Figure 23.1). Rey likened this to a baby kangaroo in its mother’s pouch. A mother can share the role with others, especially the baby’s father.
In a Cochrane systematic review of 16 RCTs (2518 babies) kangaroo care reduced mortality at 40–41 weeks (typical RR, 0.60, 95% CI 0.39–0.93), reduced nosocomial infections (typical RR 0.42, 95% CI 0.24–0.73), reduced hypothermia by 79% and reduced length of hospital stay by a mean of 2.4 days.66
Kangaroo care is a cheap and highly effective intervention in resource-poor countries where neonatal special and intensive care is unavailable and/or unaffordable (Table 23.3). Its use has not been studied adequately in Western countries.
23.3.4 Delayed cord clamping
Delayed umbilical cord clamping for pre-term deliveries improves haematocrit and circulation and reduces intraventricular haemorrhage (IVH). In an RCT of babies delivered <;32 weeks of gestation, delayed cord clamping (at 30–45 seconds) reduced blood culture-proven infection compared to immediate (5–10 seconds) clamping (1 of 36 delayed vs. 8 of 36 immediate clamping, p = 0.03). Infants with sepsis had significantly lower haematocrits at birth.69 An under-powered Israeli study of 35 pre-term infants (29 <;1500 g) found no effect on the incidence neonatal infections.70
A Cochrane systematic review of timing of cord clamping on maternal and neonatal outcomes did not examine NEC or neonatal infection.71 Another Cochrane systematic review looked at the effect of delayed cord clamping on NEC but not on neonatal infection.72 At least one large RCT is underway which will compare neonatal infections in pre-term babies following immediate or delayed cord clamping.
23.4 Immunomodulatory interventions
23.4.1 Polyclonal intravenous immunoglobulin
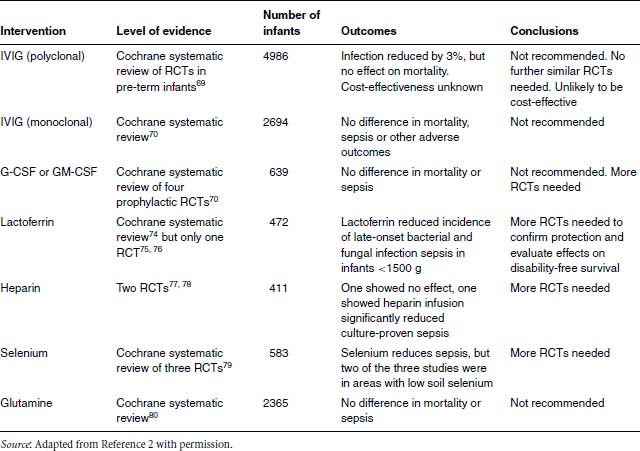
A Cochrane systematic review of polyclonal IVIG to prevent neonatal infections identified 19 RCTs in 4986 pre-term or very low birth-weight infants.73 There was a significant reduction in one or more episodes of any bloodstream infection (RR 0.85, 95% CI 0.74, 0.98). The absolute risk difference was small: 3% for any proven bloodstream infection and 4% for any ‘serious infection’. There was no reduction in mortality, NEC, IVH or length of hospital stay.73 The reduction in serious infection, without reduction in other adverse outcomes, was judged by the Cochrane review authors as being of marginal clinical significance. The NNT was 33 to prevent one bloodstream infection and 25 to prevent one ‘serious infection’. The authors concluded, unusually for a Cochrane review, that further studies were not warranted and advised basing decisions on use on the costs and values assigned to the clinical outcomes.73 There are no published cost-effectiveness analyses of prophylactic IVIG, which is almost certainly not cost-effective compared to other effective health-care interventions and cannot be recommended (Table 23.4).
Table 23.4 Immunomodulatory interventions to prevent neonatal infection.
23.4.2 Anti-staphylococcal immunoglobulins
Staphylococci are of major importance in neonatal infection. CoNS cause over half of all late-onset infections in Western countries. MSSA and MRSA infections are important late-onset pathogens in all countries and important early-onset pathogens in many developing countries. Researchers have developed various type-specific antibodies targeted at different antigenic markers of the Staphylococcus.81–83
A Cochrane systematic review81 of three RCTs of anti-staphylococcal IVIG compared to placebo showed no difference in sepsis, mortality or other adverse outcomes in two RCTs (n = 2488) of INH-A21 (Veronate), an antibody to ‘microbial surface components recognizing adhesive matrix molecules’ (RR 1.07; 95% CI 0.94–1.22), and in an RCT (n = 206) of Altastaph, which targets capsular polysaccharide antigens (RR 0.86; 95% CI 0.32–2.28). Further RCTs are in progress (Table 23.4).
23.4.3 Colony-stimulating factors
A Cochrane systematic review of G-CSF or GM-CSF in neonates found three prophylaxis studies (359 neonates) which did not show a significant reduction in mortality (RR 0.59, 95% CI 0.24–1.44).84 The primary outcome of sepsis was not evaluable because of unsatisfactory definitions of infection. In one study the incidence of systemic infection in a subgroup of infants <;32 weeks of gestation who were neutropoenic or at high risk of developing post-natal neutropoenia was reduced from 53% to 31%, but the numbers were small (n = 31) and the difference not statistically significant (RR 0.59, 95% CI 0.25–1.39).85
A subsequent multi-centre trial randomized 280 neonates <;31 weeks of gestation with low birth weight (<;10th percentile) within 72 hours of birth to receive GM-CSF for 5 days or standard care.86 Although neutrophil counts rose more rapidly in the GM-CSF babies, there was no significant difference in sepsis-free survival for all infants. A meta-analysis of this and previous published trials showed no evidence of survival benefit.86
Colony-stimulating factors are not indicated for prophylaxis to prevent systemic infection in high-risk neonates (Table 23.4).
23.4.4 Lactoferrin
Lactoferrin is a naturally occurring glycoprotein with antimicrobial activity (see Chapter 6).74,87,88
A Cochrane systematic review74 found one eligible RCT of bovine lactoferrin.75 This Italian trial randomized 472 infants <;1500 g to oral lactoferrin alone, lactoferrin plus the probiotic Lactobacillus rhamnosus GG or placebo from birth until 30 days. Late-onset sepsis was significantly lower in the groups that received lactoferrin alone (RR 0.34, 95% CI 0.17–0.70) or lactoferrin in combination with L. rhamnosus GG (RR 0.27, 95% CI 0.12–0.60).75 The incidence of NEC was significantly reduced with lactoferrin and L. rhamnosus GG (RR 0.05, 95% CI 0.00–0.90), but not oral lactoferrin alone (RR 0.33, 95% CI 0.09–1.17). No adverse effects due to lactoferrin were observed. A subsequent secondary analysis of the data found that invasive fungal infection was significantly lower with bovine lactoferrin alone (0.7%) or plus L. rhamnosus GG (2%) than with placebo (7.7%).76
One double-blind RCT found that formula-fed infants whose formula was supplemented with bovine lactoferrin in the first 12 months of life had significantly fewer respiratory infections than unsupplemented formula-fed infants (0.15 vs. 0.5 episodes/year).89
More studies of lactoferrin are in progress (Table 23.4).
23.4.5 Heparin
Heparin can prolong the duration of central venous catheters. An RCT found that infants who had heparin infusion had a slightly higher though not statistically different rate of suspected or proven sepsis than controls (10 of 100 vs. 6 of 101).77
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



