Emily Ko
Lisa Abaid
Kevin Schuler
The successful outcome of gynecologic surgery is based on thorough evaluation, careful preoperative preparation, and attentive postoperative care. This chapter discusses approaches to the general perioperative management of patients undergoing major gynecologic surgery with specific medical problems that could complicate the surgical outcome.
Medical History and Physical Examination
Gynecologic surgery should be undertaken only after gaining a thorough understanding of a patient’s medical history and performing a complete physical examination.
Table 22.1 Potential Effects of Common Herbal and Dietary Supplements
| Herb/Dietary Supplement | Potential Perioperative Effect |
| Aconite | Potential ventricular arrhythmias |
| Aloe | May potentiate thiazides |
| Black cohosh | May potentiate hypotensive effects |
| Danshen | May cause bleeding |
| Dong quai | May cause bleeding |
| Echinacea | Allergic reactions; decreased effectiveness of immunosuppressants |
| Ephedra/ma huang | Risk of myocardial ischemia and stroke from tachycardia and hypertension; ventricular arrhythmias with halothane; long-term use may cause intraoperative hemodynamic instability; life-threatening interaction with monoamine oxidase inhibitors, anesthesia, potential for withdrawal |
| Licorice | May cause hypertension and hypokalemia |
| Senna | May cause electrolyte imbalance |
| St. John’s wort | Induction of cytochrome p450 enzyme; excessive sedation and delayed emergence from general anesthesia; potential serotonin syndrome if used in combination with other serotoneregic agents |
| Valerian | Excessive sedation and delayed emergence from general anesthesia; benzodiazepine-like acute withdrawal |
| Yerba mate | May cause hypertension or hypotension and excess sympathetic nervous system stimulation |
Laboratory Evaluation
“Routine” preoperative laboratory testing of healthy women is to be discouraged as abnormal results are infrequent and are rarely of consequence in the surgical or anesthetic management of the patient (3). Despite well-established guidelines, approximately 90% of patients undergo unnecessary testing in a major university medical center (4). The selection of appropriate preoperative laboratory studies should depend on the type of the anticipated surgical procedure and the patient’s medical status.
Imaging of adjacent organ systems should be undertaken in individual cases as follows:
Preoperative Discussion and Informed Consent
The preoperative discussion should include a description of the surgical procedure, its expected outcome and risks and is the basis for obtaining signed informed consent (8,9). Informed consent is an educational process for the patient and her family and fulfills the physician’s need to convey information in understandable terms. The items listed in Table 22.2 should be discussed, and, after each item, the patient and family should be invited to ask questions. Documentation of the discussion is an important component of the patient’s record that the physician should always include with the preprinted consent form.
Table 22.2 Outline of Key Points of the Preoperative Informed Consent Discussion
1. The nature and extent of the disease process 2. The extent of the actual operation proposed and the potential modifications of the operation, depending on intraoperative findings 3. The anticipated benefits of the operation, with a conservative estimate of successful outcome 4. The risks and potential complications of the surgery 5. Alternative methods of therapy and the risks and results of those alternative methods of therapy 6. The results likely if the patient is not treated |
Following are components of the informed consent process:
General Considerations
Nutrition
Young patients undergoing elective gynecologic surgery have adequate nutritional stores and, for the most part, do not require nutritional support. All patients should have a nutritional assessment, especially elderly patients and those undergoing gynecologic cancer surgery or other major gynecologic procedures in which a prolonged postoperative recovery is expected. Nutritional status should be reassessed at regular intervals postoperatively until the patient successfully returns to a regular diet.
A nutritional assessment includes a careful history and physical examination, which are the most useful, reliable, and cost-effective methods of determining a patient’s nutritional status. In particular, information about recent weight loss, dietary history, fad diets, extreme exercise, or anorexia or bulimia should be elucidated. Physical evidence of malnutrition includes temporal wasting, muscle wasting, ascites, and edema. Accurate height and weight measurements should be obtained and an ideal body weight, percentage ideal body weight, and percentage usual body weight may be calculated. Many Internet-based body weight calculators are available. A variety of techniques were developed to determine a patient’s nutritional state; however, many methods lack clinical utility outside of a research setting. Anthropometric measurements of skin-fold thickness and arm-muscle circumference provide an estimate of total body fat and lean muscle mass.
The calculated body mass index (BMI) can be used as a surrogate marker for nutritional status. The BMI is calculated as body weight in kilograms divided by the height in square centimeters. A BMI less than 22 increases the risk of malnutrition, and a BMI less than 19 gives clear evidence of malnutrition (10).
Patients who have lost less than 6% of their ideal body weight do not need preoperative nutritional intervention. However, patients who have lost more than 10% of their ideal body weight in 6 months meet the definition of severely malnourished and should be considered for preoperative intervention (11). Patients who have lost between 6% and 10% should undergo further studies to determine if preoperative intervention is needed. Laboratory assessments of albumin, transferrin, and prealbumin may be obtained in addition to the routine preoperative tests. The degree of malnutrition can in part be determined by serum concentrations of albumin, transferrin, and prealbumin. The levels of these serum proteins are greatly influenced by the patient’s level of hydration. Prealbumin has the shortest half-life, at 2 to 3 days, and levels of this protein are depressed very early in comparison with serum transferrin and albumin, which have half-lives of 8 and 20 days, respectively (12). Serum albumin is a substitute for the Prognostic Nutritional Index, which is a time-consuming calculation, in assessing malnutrition in women with gynecologic malignancies (13). A serum albumin level of 3.5 to 5.0 is in the normal range, 2.8 to 3.4 is considered to indicate mild malnutrition, 2.1 to 2.7 moderate malnutrition, and less than 2.1 severe malnutrition (14). Hypoalbuminemia is correlated with morbidity, mortality, and increased postoperative complication rates in data from the National Surgical Quality Improvement Program (15). Decisions regarding the need for nutritional support should be based on several individualized factors. These factors include the patient’s prior nutritional state, the anticipated length of time in which the patient will not be able to eat, the severity of surgery, and the likelihood of complications. The nutritional assessment should determine whether the cause of the malnutrition is increased enteral loss (malabsorption, intestinal fistula), decreased oral intake, increased nutritional requirements as a result of hypermetabolism (sepsis, malignancy), or a combination of these factors. Severe malnutrition, if not corrected, can further complicate the postoperative problem by causing altered immune function, chronic anemia, impaired wound healing, and eventually multiple organ system failure and death.
The patient’s nutritional requirements are increased by surgery for several reasons. First, there is a period following surgery during which oral intake is not allowed or is very limited. In addition, the operation itself causes increased protein catabolism, increased energy requirements, and a negative nitrogen balance. If the surgery is uncomplicated and the patient is without food for less than 7 days, this response is limited and patients usually recover without the need for nutritional support. An adequate diet is defined as providing 75% of estimated caloric and protein needs. Therefore, if an adequate oral diet is not expected for 7 to 10 days, perioperative nutritional support may be required to avoid progressive malnutrition and associated complications (14). Perioperative nutritional support reduces operative morbidity and decreases the length of hospitalization when commenced early in the postoperative course. Patients with either normal nutritional indices or mild or moderate malnutrition, who will be undergoing surgical procedures likely to require a prolonged catabolic period of more than 7 to 10 days, should have enteral or parenteral nutrition instituted in the early postoperative period as soon as the patient is hemodynamically stable. This type of management should be strongly considered in patients undergoing pelvic exenterations, urinary diversions, or multiple enterectomies (16). Preoperative nutritional support is indicated for patients who have significant preexisting malnutrition or require major elective surgery. According to the American Society for Parenteral and Enteral Nutrition (ASPEN) guidelines, evidence-based medicine supports the use of preoperative nutritional support for 7 to 14 days in moderately to severely malnourished patients undergoing major nonemergent gastrointestinal surgery (12). A Veterans Affairs Total Parenteral Nutrition Cooperative Study found that severely malnourished patients preconditioned with total parenteral nutrition (TPN) had fewer complications than did control patients, excluding infectious complications (17). In a meta-analysis review of 22 studies of preoperative TPN use, a 10% decrease in postoperative complications occurred in TPN-supported patients (18). In a prospective trial including 108 women with ovarian cancer who underwent surgical cytoreduction, 88 patients had prealbumin levels less than 18 mg/dL and 24 had prealbumin levels less than 10 mg/dL (19). All postoperative mortality (23%) and 61.5% of all complications occurred in women with prealbumin less than 10 mg/dL. Women who received preoperative TPN and had prealbumin levels that increased to greater than 10 mg/dL did not have significantly increased complications. These findings lend support to consideration of preoperative TPN or neoadjuvant chemotherapy with interval cytoreduction when the nutritional status improves. These findings are encouraging but not supported by all studies or meta-analyses (20). If preoperative TPN is prescribed, it should be tapered and stopped at midnight before surgery, restarted 24 to 72 hours after the procedure, and continued until the patient is able to meet nutritional requirements.
ASPEN guidelines do not support the routine use of nutritional support in the immediate postoperative period for patients undergoing major gastrointestinal surgery; however, the guidelines do indicate a role for nutritional support postoperatively in patients in whom oral intake will be inadequate for 7 to 10 days (12). Clinical trials demonstrate that TPN can improve nutritional status as measured by biochemical assays, immune function, and nitrogen balance. The effect of TPN on clinical outcome is less well established. Despite what seems reasonable, based on common sense and preoperative nutritional parameters, the data do not support TPN for mild to moderately malnourished patients. With severe malnutrition, preoperative TPN seems to be beneficial and should be instituted.
Route of Administration
After the decision is made that nutritional support is required, the appropriate route of administration must be determined. Enteral nutrition should be considered primarily because it is easy to deliver, associated with the fewest complications, linked to enhanced wound healing, and relatively inexpensive (21). Contraindications to this route of delivery include intestinal obstruction, gastrointestinal bleeding, and diarrhea. Many types of preparations are commercially available and can be chosen based on their caloric content, fat content, protein content, osmolality, viscosity, and price. Depending on the patient’s problem, the route of delivery may be through a Dobhoff feeding tube, a gastrostomy tube, or a feeding jejunostomy tube (22). If the gastrointestinal tract is unusable for more than 7 days postoperatively, TPN should be implemented.
Total parenteral nutrition must be delivered through a central vein and has wide acceptance as a means of providing nutritional support for surgically ill patients. It must be delivered through a subclavian or internal jugular vein, and the catheter must be placed using meticulous sterile surgical technique. Only intravenous access lines in the right atrium, superior vena cava, or inferior vena cava can be truly deemed central lines (23). Proper daily care is required to avoid infectious complications. When managed by an experienced team, the most frequent complication, infection, can be minimized (24).
Composition of Total Parenteral Nutrition Solutions
AEE (women) = [655.10 + 9.56 Weight (kg) + 1.85 Height (cm) – 4.68 Age (yrs)] × (activity factor) × (injury factor)
Activity factor: confined to bed (1.2), out of bed (1.3).
Injury factor: minor surgery (1.2), skeletal trauma (1.3), major sepsis (1.6), severe burn (2.1).
Alternatively, daily caloric requirements can be met by giving the patient 35 kcal/kg/day for maintenance and 45 kcal/kg/day for anabolic states.
Fluid and Electrolytes
Water constitutes approximately 50% to 55% of the body weight of the average woman. Two-thirds of this water is contained in the intracellular compartment. One-third is contained in the extracellular compartment, of which one-fourth is contained in plasma, and the remaining three-fourths is in the interstitium.
Osmolarity, or tonicity, is a property derived from the number of particles in a solution. Sodium and chloride are the primary electrolytes contributing to the osmolarity of the extracellular compartment. Potassium and, to a lesser extent, magnesium and phosphate are the major intracellular electrolytes. Water flows freely between the intracellular and the extracellular spaces to maintain osmotic neutrality throughout the body. Any shifts in osmolarity in any fluid spaces within the body are accompanied by corresponding shifts in free water from spaces of lower to higher osmolarity, thus maintaining equilibrium.
The average adult daily fluid maintenance requirement is approximately 30 mL/kg/day, or 2,000 to 3,000 mL per day (26). This level is offset partially by insensible losses of 1,200 mL per day, which include losses from the lungs (600 mL), skin (400 mL), and gastrointestinal tract (200 mL). Urinary output from the kidney accounts for the remainder of the fluid loss, and this output will vary depending on total body intake of water and sodium. Approximately 600 to 800 mOsm of solute are excreted by the kidneys daily. Healthy kidneys can concentrate urine up to approximately 1,200 mOsm and, therefore, the minimum output can range between 500 and 700 mL per day. The maximal urine output of the kidney can be as high as 20 L per day, as seen in patients with diabetes insipidus. In healthy individuals, the kidney adjusts output commensurate with daily fluid intake.
The major extracellular buffer used in the acid-base balance is the bicarbonate-carbonic acid system: CO2 + H2O ↔ H2CO3 ↔ H+ + HCO3− (27). Typically, the body will maintain a bicarbonate-to-carbonic acid ratio of 20:1 to maintain an extracellular pH of 7.4. Both the lung and the kidney play integral roles in the maintenance of normal extracellular pH via retention or excretion of carbon dioxide and bicarbonate. Under conditions of alkalosis, minute ventilation decreases and renal excretion of bicarbonate increases to restore the normal ratio of bicarbonate to carbonic acid; the opposite occurs with acidosis.
Ultimately, the kidney plays the most important role in fluid and electrolyte balance through excretion and retention of water and solute. Circulating antidiuretic hormone and aldosterone help modulate the process. Serum osmolarity affects hypothalamic release of antidiuretic hormone and aldosterone secretion in response to renal perfusion. Under states of dehydration or hypovolemia, serum antidiuretic hormone levels increase, leading to increased resorption of water in the distal tubule of the kidney. Increased aldosterone release promotes increased sodium and water retention. The opposite occurs in states of fluid excess. Individuals with normal renal function and circulating antidiuretic hormone and aldosterone levels maintain normal serum osmolarity and electrolyte composition, despite daily fluctuations of fluid and electrolyte intake.
Various disease states can alter the normal fluid and electrolyte homeostatic mechanisms, making perioperative fluid and electrolyte management more difficult. Patients with intrinsic renal disease are unable to excrete solute and to maintain acid-base balance. In patients undergoing the stress of chronic starvation or severe illness, there may be an inappropriately high level of circulating antidiuretic hormone and aldosterone, resulting in fluid and sodium retention. With severe cardiac disease, secondary renal hypoperfusion can lead to increased aldosterone synthesis and increased sodium and water retention by the kidney. Patients with severe diabetes can have significant osmotic diuresis as well as acid-base dysfunction secondary to circulating keto acids. Treatment of renal, cardiac, or endocrine disorders preoperatively is imperative and often will rectify fluid and electrolyte abnormalities.
Special attention is warranted in the elderly patient undergoing surgery. Normal physiological changes associated with aging can increase the likelihood of fluid and electrolyte disorders. These changes include decreased glomerular filtration rate, decreased urinary concentrating ability, and narrowed limits for excretion of water and electrolytes (28). Fluid and electrolyte management in the perioperative period requires knowledge of the daily fluid and electrolyte requirements for maintenance, replacement of ongoing fluid and electrolyte losses, as well as correction of any existing abnormalities.
Fluid and Electrolyte Maintenance Requirements
The body adjusts to higher and lower volumes of intake by changes in plasma tonicity. Alterations in plasma tonicity induce adjustments in circulating antidiuretic hormone levels, which ultimately regulate the amount of water retained in the distal tubule of the kidney. In the preoperative and the early postoperative periods, it is usually necessary to replace only sodium and potassium. Chloride is automatically replaced, concomitant with sodium and potassium, because chloride is the usual anion used to balance sodium and potassium in electrolyte solutions. There are various commercially available solutions containing 40 mmol of sodium chloride, with smaller amounts of potassium, calcium, and magnesium, designed to meet the requirements of a patient who is receiving 3 L of intravenous fluids per day. The daily requirement can be met by any combination of intravenous fluids. For example, 2 L of D5 (5% dextrose)/0.45 normal saline (7 mEq sodium chloride each), supplemented with 20 mEq of potassium chloride, followed by 1 L of D5W (5% dextrose in water) with 20 mEq of potassium chloride, would suffice.
Fluid and Electrolyte Replacement
Fluid and electrolyte losses beyond the daily average must be replaced by appropriate solutions. The choice of solutions for replacement depends on the composition of the fluids lost. Often, it is difficult to measure free water loss, particularly in patients who have high losses from the lungs, skin, or the gastrointestinal tract. Weighing these patients daily can be very useful. Up to 300 g of weight loss daily can be attributable to weight loss from catabolism of protein and fat in the patient who is taking nothing by mouth (26). Any loss beyond this level represents fluid loss, which should be replaced accordingly.
Patients with a high fever can have increased pulmonary and skin loss of free water, sometimes in excess of 2 to 3 L per day. These losses should be replaced with free water in the form of D5W. Perspiration typically has one-third the osmolarity of plasma and can be replaced with D5W or, if the loss is excessive, with D5/0.25 normal saline.
Patients with acute blood loss need replacement with appropriate isotonic fluid or blood or both. There is a wide range of plasma volume expanders, including albumin, dextran, and hetastarch solutions, that contain large molecular weight particles (<50 kDa molecular weight). These particles are slow to exit the intravascular space, and about one-half of the particles remain after 24 hours. Controversy exists over the ideal strategy for intravascular volume replacement (29). A systematic review of 25 randomized clinical trials demonstrated preserved renal function and reduced intestinal edema in surgical patients receiving hyperoncotic albumin solutions, as compared with control fluids (30). Meta-analyses on the use of human albumin and crystalloids versus colloids in fluid resuscitation did not show a benefit in mortality rates (31,32). Caution is required in interpreting results from these pooled controlled trials because mortality outcome was not the end point of most of the studies, and publication bias is a limitation. Possible side effects with synthetic colloid solutions include adverse affects on hemostasis, severe anaphylactic reactions, and impairment of renal function (29). These solutions are expensive and for most cases, simple replacement with 0.9 normal saline or lactated Ringer’s solution will suffice. One-third of the volume of lactated Ringer’s solution or normal saline typically will remain in the intravascular space and the remainder goes to the interstitium.
Appropriate replacement of gastrointestinal fluid loss depends on the source of fluid loss in the gastrointestinal tract. Gastrointestinal secretions beyond the stomach and up to the colon are typically isotonic with plasma, with similar amounts of sodium, slightly lower amounts of chloride, slightly alkaline pH, and more potassium (in the range of 10 to 20 mEq/L). Under normal conditions, stool is hypotonic. However, under conditions of increased flow (i.e., severe diarrhea), stool contents are isotonic with a composition similar to that of the small bowel contents. Gastric contents are typically hypotonic, with one-third the sodium of plasma, increased amounts of hydrogen ion, and low pH.
In patients who have gastric outlet obstruction, nausea, and vomiting, or who undergo nasogastric suction, appropriate replacement of gastric secretions can be provided with a solution such as D5/0.45 normal saline with 20 mEq/L of potassium. Potassium supplementation is particularly important to prevent hypokalemia in these patients, whose kidneys attempt to conserve hydrogen ions in the distal tubule of the kidney in exchange for potassium ions.
In patients with bowel obstruction, 1 to 3 L of fluid can be sequestered daily in the gastrointestinal tract. This fluid should be replaced with isotonic saline or lactated Ringer’s solution. Similarly, patients with enterocutaneous fistulas or new ileostomies should receive replacement with isotonic fluids.
Correction of Existing Fluid and Electrolyte Abnormalities
Patients who have fluid or electrolyte abnormalities preoperatively can pose a diagnostic challenge. The correct diagnosis and therapy is contingent on a correct assessment of total body fluid and electrolyte status. The management of hyponatremia, for example, may be either fluid restriction or fluid replacement. The choice of treatment depends on whether there is overall extracellular fluid excess and normal body sodium stores or decreased overall total body sodium stores and extracellular fluid. A detailed history is necessary to disclose any underlying medical illness and to assess the amount and duration of any abnormal fluid losses or intake. Initial evaluation should include an assessment of hemodynamic, clinical, and urinary parameters to determine the overall level of hydration as well as the fluid status of the extracellular fluid compartment. The patient who has good skin turgor, moist mucosa, stable vital signs, and good urinary output is well hydrated. Nonpitting edema is indicative of extracellular fluid excess, whereas patients with orthostasis, sunken eyes, parched mouth, and decreased skin turgor have extracellular volume contraction. A patient’s overall extracellular fluid status does not always reflect the hydration status of the intravascular compartment. A patient can have increased interstitial fluid and yet be intravascularly dry, requiring replacement with isotonic fluid.
The laboratory workup for patients who may have preexisting fluid problems should include assessment of blood hematocrit, serum chemistry, glucose, blood urea nitrogen (BUN) and creatinine, urine osmolarity, and urine electrolyte levels. Serum osmolarity is mainly a function of the concentration of sodium and is given by the following equation:
2[Na+] + glucose (mg/dL)/18 +BUN (mg/dL)/2.8
Normal serum osmolarity is typically 290 to 300 mOsm. Blood hematocrit will rise or fall inversely at a rate of 1% per 500-mL alteration of extracellular fluid volume. The BUN:creatinine ratio is typically 10:1 but will rise to a ratio of greater than 20:1 under conditions of extracellular fluid contraction. Under conditions of extracellular fluid deficit, urine osmolarity will typically be high (>400 mOsm), whereas urine sodium concentration is low (<15 mEq/L), indicative of an attempt by the kidney to conserve sodium. Under conditions of extracellular fluid excess or in cases of renal disease in which the kidney has impaired ability to retain sodium and water, urine osmolarity will be low and urine sodium will be high (>30 mEq/L). Changes in sodium can give insight into the degree of extracellular fluid excess or deficit. In the average person, the serum sodium rises by 3 mmol/L for every liter of water deficit and falls by 3 mmol/L for each liter of water excess. One must be careful in making these estimates because patients with prolonged water and electrolyte loss can have low serum sodium levels and marked water deficits.
Specific Electrolyte Disorders
Hyponatremia
Because sodium is the major extracellular cation, shifts in serum sodium levels are usually inversely correlated with the hydration state of the extracellular fluid compartment. The pathophysiology of hyponatremia is usually expansion of body fluids leading to excess total body water (27,33). Symptomatic hyponatremia usually does not occur until the serum sodium is below 120 to 125 mEq/L. The severity of the symptoms (nausea, vomiting, lethargy, seizures) is related more to the rate of change of serum sodium than to the actual serum sodium level.
Hyponatremia in the form of extracellular fluid excess can be seen in patients with renal or cardiac failure and in conditions such as nephrotic syndrome, in which total body salt and water are increased, with a relatively greater increase in the latter. Administration of hypertonic saline to correct the hyponatremia would be inappropriate in this setting. The treatment should include, in addition to correcting the underlying disease process, water restriction with diuretic therapy. Inappropriate secretion of antidiuretic hormone (ADH) can occur with head trauma, pulmonary or cerebral tumors, and states of stress. The abnormally elevated ADH results in excess water retention. Treatment includes water restriction and, if possible, correction of the underlying cause. Demeclocycline, a tetracycline antibiotic, is effective in this disorder via its action in the kidney. The introduction of vasopressin receptor antagonists, such as tolvaptan, may replace demeclocycline as the drug of choice for the syndrome of inappropriate ADH secretion (34).
Inappropriate replacement of body salt losses with water alone will result in hyponatremia. This situation will typically occur in patients who lose large amounts of electrolytes secondary to vomiting, nasogastric suction, diarrhea, or gastrointestinal fistulas, and who received replacement with hypotonic solutions. Simple replacement with isotonic fluids and potassium will usually correct the abnormality. Rarely, rapid correction of the hyponatremia is necessary, in which case hypertonic saline (3%) can be administered. Hypertonic saline should be administered very cautiously to avoid a rapid shift in serum sodium, which will induce central nervous system dysfunction.
Hypernatremia
Hypernatremia is an uncommon condition that can be life-threatening if severe (serum sodium greater than 160 mEq/L). The pathophysiology is extracellular fluid deficit. The resultant hyperosmolar state leads to decreased water volume in cells in the central nervous system, which, if severe, can cause disorientation, seizures, intracranial bleeding, and death. The causes include excessive extrarenal water loss, which can occur in patients who have a high fever, have undergone tracheostomy in a dry environment, or have extensive thermal injuries; who have diabetes insipidus, either central or nephrogenic; and who have iatrogenic salt loading. The treatment involves correction of the underlying cause (correction of fever, humidification of the tracheostomy, administration of desmopressin for control of central diabetes insipidus) and replacement with free water either by the oral route or intravenously with D5W. As with severe hyponatremia, marked hypernatremia should be corrected slowly, no faster than 10 mEq per day, unless the patient is symptomatic from severe acute hypernatremia (35).
Hypokalemia
Hypokalemia may be encountered preoperatively in patients with significant gastrointestinal fluid loss (prolonged emesis, diarrhea, nasogastric suction, intestinal fistulas) and marked urinary potassium loss secondary to renal tubular disorders (renal tubular acidosis, acute tubular necrosis, hyperaldosteronism, prolonged diuretic use). It can arise from prolonged administration of potassium-free parenteral fluids in patients who are restricted from ingesting anything by mouth. The symptoms associated with hypokalemia include neuromuscular disturbances, ranging from muscle weakness to flaccid paralysis, and cardiovascular abnormalities, including hypotension, bradycardia, arrhythmias, and enhancement of digitalis toxicity. These symptoms rarely occur unless the serum potassium level is less than 3 mEq/L. The treatment is potassium replacement. Oral therapy is preferable in patients who are on an oral diet. If necessary, potassium replacement can be given intravenously in doses that should not exceed 10 mEq per hour.
Hyperkalemia
Hyperkalemia is encountered infrequently in preoperative patients. It is usually associated with renal impairment but can be seen in patients who have adrenal insufficiency, are taking potassium-sparing diuretics, and have marked tissue breakdown such as that occurring with crush injuries, massive gastrointestinal bleeding, or hemolysis. The clinical manifestations are mainly cardiovascular. Marked hyperkalemia (potassium >7 mEq/L) can result in bradycardia, ventricular fibrillation, and cardiac arrest. The treatment chosen depends on the severity of the hyperkalemia and whether there are associated cardiac abnormalities detected with electrocardiography. Calcium gluconate (10 mL of a 10% solution), given intravenously, can offset the toxic effects of hyperkalemia on the heart. One ampule each of sodium bicarbonate and D5W, with or without insulin, will cause a rapid shift of potassium into cells. Over the longer term, cation exchange resins such as sodium polystyrene sulfate (Kayexalate), taken orally or by enema, will bind and decrease total body potassium. Hemodialysis is reserved for emergent conditions in which other measures are not sufficient or have failed (35).
Postoperative Fluid and Electrolyte Management
Several hormonal and physiologic alterations in the postoperative period may complicate fluid and electrolyte management. The stress of surgery induces an inappropriately high level of circulating ADH. Circulating aldosterone levels are increased, especially if sustained episodes of hypotension occurred either intraoperatively or postoperatively. The elevated levels of circulating ADH and aldosterone make postoperative patients prone to sodium and water retention.
Total body fluid postoperative volume may be altered significantly. First, 1 mL of free water is released for each gram of fat or tissue that is catabolized and, in the postoperative period, several hundred milliliters of free water are released daily from tissue breakdown, particularly in the patient who has undergone extensive intra-abdominal dissection and who is restricted from ingesting food and fluids by mouth. This free water is often retained in response to the altered levels of ADH and aldosterone. Second, fluid retention is further enhanced by third spacing, or sequestration of fluid in the surgical field. The development of an ileus may result in an additional 1 to 3 L of fluid per day being sequestered in the bowel lumen, bowel wall, and peritoneal cavity.
In contrast to renal sodium homeostasis, the kidney lacks the capacity for retention of potassium. In the postoperative period, the kidneys continue to excrete a minimum of 30 to 60 mEq/L of potassium daily, irrespective of the serum potassium level and total body potassium stores (27). If this potassium is not replaced, hypokalemia may develop. Tissue damage and catabolism during the first postoperative day usually result in the release of sufficient intracellular potassium to meet the daily requirements. Beyond the first postoperative day, potassium supplementation is necessary.
Correct maintenance of fluid and electrolyte balance in the postoperative period starts with the preoperative assessment, with emphasis on establishing normal fluid and electrolyte parameters before surgery. Postoperatively, close monitoring of daily weight, urine output, serum hematocrit, serum electrolytes, and hemodynamic parameters will yield the necessary information to make correct adjustments in crystalloid replacement. The normal daily fluid and electrolyte requirements must be met and any unusual fluid and electrolyte losses, including those from the gastrointestinal tract, lungs, or skin, must be compensated. After the first few postoperative days, third-space fluid begins to return to the intravascular space, and ADH and aldosterone levels revert to normal. The excess fluid retained perioperatively is mobilized and excreted through the kidneys, and exogenous fluid requirements decrease. Patients with inadequate cardiovascular or renal reserve are prone to fluid overload during this time of third-space reabsorption, especially if intravenous fluids are not appropriately reduced.
The most common fluid and electrolyte disorder in the postoperative period is fluid overload. Fluid excess can occur concomitantly with normal or decreased serum sodium. Large amounts of isotonic fluids are usually infused intraoperatively and postoperatively to maintain blood pressure and urine output. Because the infused fluid is often isotonic with plasma, it will remain in the extracellular space. Under such conditions, serum sodium will remain within normal levels. Fluid excess with hypotonicity (decreased serum sodium) can occur if large amounts of isotonic fluid losses (e.g., blood and gastrointestinal tract) are inappropriately replaced with hypotonic fluids. The predisposition toward retention of free water in the immediate postoperative period compounds the problem. An increase in body weight occurs concomitantly with the fluid expansion. In the patient who is not allowed anything by mouth, catabolism should induce a daily weight loss as great as 300 g per day. The patient who is gaining weight in excess of 150 g per day is in a state of fluid expansion. Simple fluid restriction will correct the abnormality. When necessary, diuretics can be used to increase urinary excretion.
States of fluid dehydration are uncommon but will occur in patients who have large daily losses of fluid that are not replaced. Gastrointestinal losses should be replaced with the appropriate fluids. Patients with high fevers should be given appropriate free water replacement, because up to 2 L per day of free water can be lost through perspiration and hyperventilation. Although these increased losses are difficult to monitor, a reliable estimate can be obtained by monitoring body weight.
Postoperative Acid-Base Disorders
A variety of metabolic, respiratory, and electrolyte abnormalities in the postoperative period can result in an imbalance in normal acid-base homeostasis, leading to alkalosis or acidosis. Changes in the respiratory rate directly affect the amount of carbon dioxide that is exhaled. Respiratory acidosis will result from carbon dioxide retention in patients who have hypoventilation from central nervous system depression. This condition can result from oversedation with narcotics, particularly in the presence of concurrent severe chronic obstructive pulmonary disease. Respiratory alkalosis can result from hyperventilation caused by excitation of the central nervous system by drugs, pain, or excess ventilator support. Numerous metabolic derangements can result in metabolic alkalosis or acidosis. Proper fluid and electrolyte replacement as well as maintenance of adequate tissue perfusion will help prevent most acid-base disorders that occur during the postoperative period.
Alkalosis
The most common acid-base disorder encountered in the postoperative period is alkalosis (27). Alkalosis is usually of no clinical significance and resolves spontaneously. Several etiologic factors may include hyperventilation associated with pain; posttraumatic transient hyperaldosteronism, which results in decreased renal bicarbonate excretion; nasogastric suction, which removes hydrogen ions; infusion of bicarbonate during blood transfusions in the form of citrate, which is converted to bicarbonates; administration of exogenous alkali; and use of diuretics. Alkalosis can be corrected with removal of the inciting cause and the correction of extracellular fluid and potassium deficits (Table 22.3). Full correction usually can be safely achieved over 1 to 2 days.
Table 22.3 Causes of Metabolic Alkalosis
| Disorder | Source of Alkali | Cause of Renal HCO Retention |
| Gastric alkalosis | ||
| Nasogastric suction | Gastric mucosa | ↓↓ECF, ↓K |
| Vomiting | ||
| Renal alkalosis | ||
| Diuretics | Renal epithelium | ↓ECF, ↓K |
| Respiratory acidosis and diuretics | ↓ECF, ↓K, ↑PCO2 | |
| Exogenous base | NaHCO3, Na citrate, Na lactate | Coexisting disorder of ECF, K, PaCO2 |
| ↓ECF, extracellular fluid depletion; ↓K, potassium depletion; ↑↑PCO2, carbon dioxide retention; NaHCO3, sodium bicarbonate; PaCO2, partial pressure of carbon dioxide, arterial. | ||
Marked alkalosis, with serum pH higher than 7.55, can result in serious cardiac arrhythmias or central nervous system seizures. Myocardial excitability is particularly pronounced with concurrent hypokalemia. Under such conditions, fluid and electrolyte replacement may not be sufficient to correct the alkalosis rapidly. Acetazolamide (250 to 500 mg) can be given orally or intravenously two to four times daily to induce renal bicarbonate diuresis. Treatment with an acidifying agent rarely is necessary and should be reserved for acutely symptomatic patients (i.e., those with cardiac or central nervous system dysfunction) or for patients with advanced renal disease. Under such conditions, hydrogen chloride (5 to 10 mEq per hour of a 100-mmol solution) can be given via a central intravenous line. Ammonium chloride can be given orally or intravenously but should not be given to patients with hepatic disease.
Acidosis
Metabolic acidosis is less common than alkalosis during the postoperative period, but acidosis can be serious because of its effect on the cardiovascular system. Under conditions of acidosis, there are decreased myocardial contractility, a propensity for vasodilation of the peripheral vasculature leading to hypotension, and refractoriness of the fibrillating heart to defibrillation (27). These effects promote decompensation of the cardiovascular system and can hinder attempts at resuscitation.
Metabolic acidosis results from a decrease in serum bicarbonate levels caused by the consumption and replacement of bicarbonate by circulating acids or the replacement by other anions such as chloride. The proper workup includes a measurement of the anion gap:
Anion gap = (Na+ + K+) – (CI− + HCO3−) = 10 to 14 mEq/L (normal)
The anion gap is composed of circulating protein, sulfate, phosphate, citrate, and lactate (36).
With metabolic acidosis, the anion gap can be increased or normal. An increase in circulating acids will consume and replace bicarbonate ion, increasing the anion gap. The causes include an increase in circulating lactic acid secondary to anaerobic glycolysis, such as that seen under conditions of poor tissue perfusion; increased ketoacids, as with cases of severe diabetes or starvation; exogenous toxins; and renal dysfunction, which leads to increased circulating sulfates and phosphates (37). The diagnosis can be established via a thorough history and measurement of serum lactate (normal <2 mmol/L), serum glucose, and renal function parameters. Metabolic acidosis in the face of a normal anion gap is usually the result of an imbalance of the ions chloride and bicarbonate, which occurs under conditions leading to excess chloride and decreased bicarbonate. Hyperchloremic acidosis can be seen in patients who underwent saline loading. Bicarbonate loss will be seen in patients with small bowel fistulas, new ileostomies, severe diarrhea, or renal tubular acidosis. In patients with marked extracellular volume expansion, which often occurs postoperatively, the relative decrease in serum sodium and bicarbonate will result in a mild acidosis. A summary of the various causes of metabolic acidosis is shown in Table 22.4.
Table 22.4 Causes of Metabolic Acidosis
| High Anion Gap | Normal Anion Gap | |
| Hyperkalemic | Hypokalemic | |
| Uremia | Hyporeninism | Diarrhea |
| Ketoacidosis | Primary adrenal failure | Renal tubular acidosis |
| Lactic acidosis | NH2Cl | Ileal and sigmoid bladders |
| Aspirin | Sulfur poisoning | Hyperalimentation |
| Paraldehyde | Early chronic renal failure | |
| Methanol | Obstructive uropathy | |
| Ethylene glycol | ||
| Methyl malonic aciduria | ||
| NH2Cl (chloramine) | ||
Adapted from Narins RG, Lazarus MJ. Renal system. In: Vandam LD, ed. To make the patient ready for anesthesia: medical care of the surgical patient, 2nd ed. Menlo Park, CA: Addison Wesley, 1984:67–114. | ||
The treatment of metabolic acidosis depends on the cause. In patients with lactic acidosis, restoration of tissue perfusion is imperative. This state can be accomplished through cardiovascular and pulmonary support as needed, oxygen therapy, and aggressive treatment of systemic infection wherever appropriate. Ketosis from diabetes can be corrected gradually with insulin therapy. Ketosis resulting from chronic starvation or from lack of caloric support postoperatively can be corrected with nutrition. In patients with normal anion gap acidosis, bicarbonate lost from the gastrointestinal tract should be replaced, excess chloride administration can be curtailed, and, where necessary, a loop diuretic can be used to induce renal clearance of chloride. Dilutional acidosis can be corrected with mild fluid restriction.
Bicarbonates should not be given unless serum pH is lower than 7.2 or severe cardiac complications secondary to acidosis are present. Close monitoring of serum potassium levels is mandatory. Under states of acidosis, potassium will exit the cell and enter the circulation. The patient with a normal potassium concentration and metabolic acidosis is actually depleted of intracellular potassium. Treatment of the acidosis without potassium replacement will result in severe hypokalemia with its associated risks. A summary of the various acid-base abnormalities and associated therapies is shown in Table 22.5.
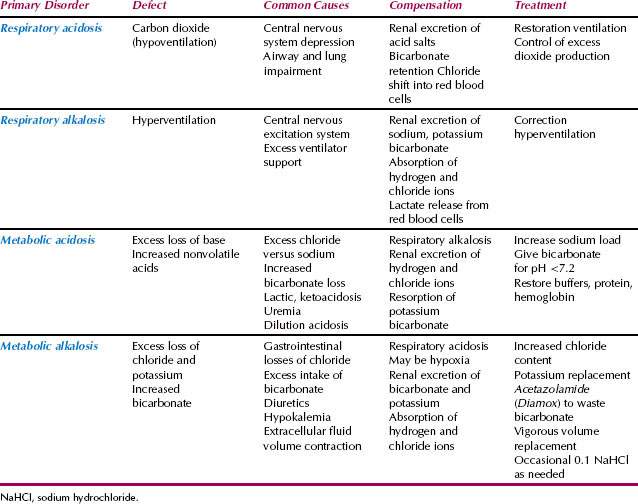
Table 22.5 Acid-Base Disorders and Their Treatment
Perioperative Pain Management
Although satisfactory analgesia is easily achievable with available methods, patients continue to suffer unnecessarily from postoperative pain. Studies consistently show that 25% to 50% of patients suffer moderate to severe pain in the postoperative period (38,39). There are several reasons for the existing inadequacies in pain management. First, patient expectations of pain relief are low and they are not aware of the extent of analgesia that they should expect. In a study of the perception of pain relief after surgery, 86% of patients had moderate to severe pain after surgery, but 70% felt that the pain was as severe as they expected (40). Second, there is a lack of formal physician training in pain management. This lack is epitomized by the commonly written order prescribing a range of narcotic to be given intramuscularly every 3 to 4 hours as needed, leaving pain management decisions to the nursing staff, with no attempt made to titrate the dose of the prescribed narcotic commensurate with individual patient requirements. Third, attitudes continue to be influenced by the common misconception that the use of narcotics in the postoperative period results in opioid dependence. In one review, 20% of nurses responding to a staff questionnaire expressed concern that the use of opioid analgesics during the postoperative period could cause addiction (40). Studies confirm that nurses administer less than one-fourth of the total dose of narcotic that is prescribed on an as-needed basis. To facilitate acute pain management and reduce the number of adverse outcomes, the American Society of Anesthesiologists established practice guidelines for acute pain management in the perioperative setting (41).
The minimal effective analgesic concentration (MEAC) refers to the serum concentration of a drug below which very little analgesia is achieved. At the MEAC, receptor and plasma concentrations of a drug are in equilibrium. Steady-state drug concentrations above the MEAC are difficult to achieve with intramuscular depot injection (42). In one study, patients receiving intramuscular injections with meperidine hydrochloride (Demerol) every 4 hours experienced marked intrapatient and interpatient variations in narcotic drug peak concentrations and in the time required to reach these peaks. As a result, serum concentrations of drug were above the MEAC an average of only 35% of each 4-hour dosing interval (43). Variable pain control following intermittent intramuscular injections is the result of inadequate, highly variable, and unpredictable blood concentrations (44). Adequate analgesia can be achieved through intramuscular or subcutaneous modes of administration, but unpredictable absorption can make titration difficult. Small intravenous boluses can be more easily titrated but may be shorter acting, requiring more frequent injections and thus intensive nursing care, whereas larger intravenous boluses may be associated with a higher incidence of central nervous system and respiratory depression. The patient-controlled analgesia (PCA) technique, which allows patients to self-administer small doses of narcotic on demand, allows titration of measured boluses of narcotic as needed to relieve pain. This technique can provide a more thorough analgesia with maintenance of steady-state drug concentrations above the MEAC.
Irrespective of the route of administration, analgesics must be front loaded to provide prompt analgesia from the start. Without front loading, attainment of the MEAC will not occur for at least three elimination half-lives of the narcotic agent that is used. After front loading, additional small boluses of narcotic can be administered until analgesia is achieved. From the total dose of drug required to achieve analgesia, maintenance drug dosages can then be determined and administered either as a continuous infusion or on a scheduled basis, so that the dose of drug administered offsets the amount that is cleared. Thereafter, prescribed doses of narcotic can be adjusted as needed.
Patient-Controlled Analgesia
Devices for administering PCA are electronically controlled infusion pumps that deliver a preset dose of narcotic into a patient’s indwelling intravenous catheter upon patient request. The devices all contain delay intervals or lockout times during which patient demands for more narcotic are not met. These devices eliminate the delay between the onset of pain and the administration of analgesic agents, a common problem inherent with on-demand analgesic orders in busy hospital wards. Patient-controlled analgesia has excellent patient acceptance. Compared with conventional intramuscular injections, serum narcotic levels have significantly lower variability in patients using PCA (42). Patients using PCA have improved analgesia, a lower incidence of postoperative pulmonary complications, and less confusion than those given intramuscular narcotics (45). The total dose of narcotic used is lower with PCA than with conventional intramuscular depot injection.
The use of PCA does not eliminate the adverse side effects of narcotics. Potentially life-threatening respiratory depression is seen in as many as 0.5% of patients using PCA. The use of a continuous narcotic infusion in addition to demand dosing is associated with a fourfold increase in respiratory depression. Elderly patients and those with preexisting respiratory compromise are at risk for respiratory depression (42).
Carefully supervised regimens using continuous infusions, on-demand intramuscular therapy, or fixed dosage schedules (every 4-hour dosing) with on-demand supplementation can have analgesic efficacy comparable with PCA. The type of close supervision required to achieve adequate on-demand analgesia without PCA is difficult to maintain. Use of PCA shortens the time between the onset of pain and the administration of pain medication, provides more continuous access to analgesics, and allows for an overall steadier state of pain control.
Epidural and Spinal Analgesia
Anesthetics and narcotics administered either in the epidural space or intrathecally are among the most potent analgesic agents available; the efficacy of these agents is greater than that provided by intravenous PCA techniques. These drugs can be administered in several ways, including a single-shot dose given by epidural or intrathecal injection, intermittent injection given either on schedule or on demand, and continuous infusion.
Because of the risk of central nervous system infections and headaches, intrathecal administration is usually limited to a single dose of narcotic, local anesthetic, or both. In comparison with epidural administration, duration of action for a single dose is increased via the intrathecal route as a result of the high concentrations of drug attained in the cerebrospinal fluid. The risk of central nervous system and respiratory depression, and systemic hypotension, is increased. The low doses of opioids required for intrathecal analgesia are sufficient to be associated with an increased risk of respiratory depression (46). Some investigators warn against the use of intrathecal spinal analgesia outside the intensive care setting.
Epidural administration is the preferred approach and provides extended (>24 hours) pain control during the postoperative period. Relative contraindications are the presence of coagulopathy, sepsis, and hypotension. Both anesthetic and narcotic agents are used with excellent efficacy. Among the anesthetic agents, bupivacaine is the most popular, providing excellent analgesia with minimal toxicity. Epidural analgesia is most suited for pain control in the lower abdomen and extremities. Potential adverse effects of epidural anesthetic agents include urinary retention, motor weakness, hypotension, and central nervous system and cardiac depression. In contrast to anesthetic agents, opioids offer excellent analgesia without accompanying sympathetic blockade. Epidural opioids tend to have a much longer duration of action, and hypotension is a rare complication. Compared with epidural anesthetics, there is a higher incidence of nausea and vomiting, respiratory depression, and pruritus (47).
Compared with analgesics administered intramuscularly or intravenously, epidural analgesia is associated with improved pulmonary function postoperatively, a lower incidence of pulmonary complications, a decrease in postoperative venous thromboembolic complications (most likely secondary to earlier ambulation), fewer gastrointestinal side effects, a lower incidence of central nervous system depression, and shorter convalescence (47). A systematic review concluded that continuous epidural anesthesia is more effective than intravenous opioid PCA in reducing postoperative pain for up to 72 hours after abdominal surgery (48). Severe respiratory depression, which occurs in less than 1% of patients, is the most serious potential complication. A lower incidence of respiratory depression occurs with the more lipophilic drugs such as fentanyl, which is quickly absorbed within the spinal cord and is less likely to diffuse to the central nervous system respiratory control centers. Pruritus, nausea, and urinary retention are common but can be managed easily and usually are of little clinical significance. Cost is perhaps the main and most limiting drawback of epidural analgesia.
Close monitoring by nursing staff is required for safe administration of epidural analgesia. An intensive care setting is not necessary. Epidural analgesics can be administered safely in a hospital ward setting under close nursing supervision, using respiratory monitoring with hourly ventilatory checks during the first 8 hours of epidural analgesia.
Nonsteroidal Anti-inflammatory Drugs
Current therapeutic strategies for perioperative pain control are largely dependent on multimodal therapy with opioid analgesics and nonsteroidal anti-inflammatory drugs (NSAIDs). The nonselective NSAID ketorolac is a potent drug that can be given orally or parenterally. Ketorolac has a slightly slower onset of activity than fentanyl but has an analgesic potency comparable to morphine. The theoretical advantages of NSAIDs over opioids include absence of respiratory depression, lack of abuse potential, decreased sedative effects, decreased nausea, early return of bowel function, and faster recovery. In clinical studies, ketorolac is found to have analgesic effects similar to those of morphine in postoperative orthopedic patients and, when used in conjunction with PCA, significantly reduced opioid requirements (49,50). Depending on the type of surgery, ketorolac has an opioid dose-sparing effect of a mean of 36% and improves analgesic control of moderate to severe pain 24 hours postoperatively (51). In the obstetric population, intravenous ketorolac is effective in reducing postoperative narcotic use after cesarean delivery (52). Although the U.S. Food and Drug Administration has not approved ketorolac for use during lactation, it was quantified in breast milk and has lower levels than ibuprofen (53).
Potential adverse effects associated with the use of NSAIDs include an increased risk of renal compromise (particularly in patients suffering from acute hypovolemia), gastrointestinal side effects, hypersensitivity reactions, and bleeding. The effects of ketorolac on bleeding are inconsistent. Studies of ketorolac on healthy volunteers showed transient increases in bleeding time and decreases in platelet aggregation, but these changes were not clinically significant (54). A retrospective cohort study showed increased risk of gastrointestinal and operative site bleeding in elderly patients receiving high doses of ketorolac, between 105 and 120 mg per day. Increased risk for all gastrointestinal bleeding was associated with use of ketorolac for more than 5 days (55). Controlled prospective studies did not show a significant increase in blood loss in patients who receive NSAIDs perioperatively. Ketorolac may be associated with elevated rates of acute renal failure when therapy exceeds 5 days (56). A meta-analyses of the use of postoperative NSAIDs in patients with normal preoperative renal function showed a clinically insignificant reduction in renal function (57). These agents should be used with extreme care, if at all, in patients with asthma, because 5% to 10% of adult patients with asthma are sensitive to aspirin and other NSAID preparations.
With the advantages of less gastrointestinal toxicity and a lack of antiplatelet effects, selective cyclooxygenase-2 (COX-2) inhibitors are a valuable option in perioperative pain management (58). Although evidence exists showing an increased risk of serious cardiovascular events associated with COX-2 inhibitors, short-term use of these agents in the perioperative setting can be considered in low-risk patients without existing cardiovascular disease (59–64).
In addition to NSAIDs, other adjuvant analgesics are being explored to minimize opioid use and the accompanying side effects, which can delay recovery. Capsaicin is a nonnarcotic that promotes release of substance P, a neurotransmitter for pain and heat, which initially results in a burning sensation, but eventually leads to substance P depletion and a reduction in pain. It is available in both topical and injectable preparations. Ketamine blocks centrally located N-methyl-d-aspartate pain receptors, and at low subanesthetic doses can reduce central sensitization caused by surgery and prevent opioid-induced hyperalgesia. At higher doses, ketamine is associated with hallucinations, dizziness, nausea, and vomiting. Gabapentin and pregabalin are nonnarcotics that prevent the release of excitatory neurotransmitters that relay pain signals. They reduce opioid requirements and are effective antihyperalgesic agents (64).
Antimicrobial Prophylaxis in Gynecologic Surgery
Gynecologic procedures often involve breaching the reproductive and gastrointestinal tracts, which harbor endogenous bacteria capable of causing polymicrobial infections in the postoperative period (Table 22.6). Despite great advances in aseptic technique and drug development, bacterial contamination of the operative site and postoperative infections are an inevitable part of the practice of gynecologic surgery. Prevention of these surgical complications includes using proper aseptic technique, minimizing tissue trauma, minimizing the amount foreign material in the surgical site, controlling diabetes, avoiding immunologic suppression, maximizing tissue oxygenation, draining blood and serum from the surgical site, and using prophylactic antibiotics. Antibiotic prophylaxis is given with the belief that antibiotics enhance the immune mechanisms in host tissues that resist infections by killing the bacteria that inoculate the surgical site during surgery (65).
Table 22.6 Bacteria Indigenous to the Lower Genital Tract
| Lactobacillus | Enterobacter agglomerans |
| Diphtheroids | Klebsiella pneumoniae |
| Staphylococcus aureus | Proteus mirabilis |
| Staphylococcus epidermidis | Proteus vulgaris |
| Streptococcus agalactiae | Morganella morganii |
| Streptococcus faecalis | Citrobacter diversus |
| α-Hemolytic streptococci | Bacteroides species |
| Group D streptococci | B. disiens |
| Peptostreptococci | B. fragilis |
| Peptococcus | B. melaninogenicus |
| Clostridium | |
| Gaffky anaerobia | |
| Escherichia coli | |
| Fusobacterium | |
| Enterobacter cloacae |
Infections in the skin or pelvis that result from gynecologic surgery (e.g., parametritis, cuff cellulitis, pelvic abscess) typically are polymicrobial in nature. These infections are complex and often involve gram-negative rods, gram-positive cocci, and anaerobes. Antibiotic prophylaxis should be sufficiently broad to cover these potential pathogens (66) (Table 22.7).
Table 22.7 Antibiotic Prophylaxis Regimens by Procedure
| Procedure | Antibiotic | Dose |
| Hysterectomy Urogynecology procedures, including those involving mesh | Cefazolina Clindamycinc plus gentamicin or quinoloned or aztreonam Metronidazolec plus gentamycin or quinoloned | 1 g or 2 g IVb 600 mg IV 1.5 mg/kg IV 400 mg IV 1 g IV 500 mg IV 1.5 mg/kg IV 400 mg IV 1 g IV |
| Hysterosalpingogram or Chromotubation | Doxycyclinee | 100 mg orally, twice daily for 5 days |
| Induced abortion/dilation and evacuation | Doxycycline Metronidazole | 100 mg orally 1 hour before procedure and 200 mg orally after procedure 500 mg orally twice daily for 5 days |
| IV, intravenously. | ||
aAlternatives include cefotetan, cefoxitin, cefuroxime, or ampicillin-sulbactam. bA 2-g dose is recommended in women with a body mass index greater than 35 or weight greater than 100 kg or 220 lb. cAntimicrobial agents of choice in women with a history of immediate hypersensitivity to penicillin. dCiprofloxacin or levofloxacin or moxifloxacin. eIf patient has a history of pelvic inflammatory disease or procedure demonstrates dilated fallopian tubes. No prophylaxis is indicated for a study without dilated tubes. | ||
| Adapted from Antibiotic prophylaxis for gynecologic procedures. American College of Obstetricians and Gynecologists Practice Bulletin No. 104, May 2009. | ||
The timing of antimicrobial prophylaxis is important. There is a relatively narrow window of opportunity for affecting outcomes (67). In the United States, it is customary to give antimicrobial prophylaxis shortly before or during the induction of anesthesia. Data revealed that a delay of 3 hours or more between the time of bacterial inoculation (i.e., skin incision) and administration of antibiotics may result in ineffective prophylaxis. Evidence indicates that for prophylaxis, one dose of antibiotic is appropriate. When the surgical procedure proceeds longer than 1 to 2 times the half-life of the drug or blood loss is greater than 1.5 L, additional intraoperative doses of antibiotics should be administered to maintain adequate levels of medication in serum and tissues (68,69). There are no data to support the continuation of prophylactic antimicrobial agents into the postoperative period for routine gynecologic procedures. For cases involving colorectal resection, a reduction in surgical site infections (SSI) was seen when antibiotics were continued for up to 24 hours. Other measures to reduce SSI incidence were taken, including tight glycemic control, maintenance of intraoperative normothermia, and placement of subcutaneous drains in obese patients (70).
Cephalosporins emerged as the most important class of antimicrobial agents for prophylaxis. These drugs have a broad spectrum and relatively low incidence of adverse reactions. Cefazolin (1 g) appears to be widely used in the United States by gynecologic surgeons because of its relatively low cost and long half-life (1.8 hours). Other cephalosporins such as cefoxitin, cefotaxime, and cefotetan commonly are used for prophylaxis. These agents appear to have a broader spectrum of activity against anaerobic bacteria and are appropriate selections when colorectal resections are possible, such as during a debulking surgery for ovarian cancer. For the majority of gynecologic procedures, there is little evidence that a clinically relevant distinction exists between cefazolin and the other agents. Morbidly obese patients, defined as having a BMI greater than 35 or weight greater than 100kg, should receive 2 g of cefazolin to achieve appropriate blood and tissue antibiotic concentrations (71).
Antimicrobial prophylaxis, although usually beneficial, is not without risk. Anaphylaxis is the most life-threatening complication from antibiotic use. Anaphylactic reactions to penicillins are reported in 0.2% courses of treatment. The fatality rate is 0.0001%. Data indicate that it is safe to administer cephalosporins to women who report a history of adverse reactions to penicillins. The incidence of adverse reactions (e.g., skin flushing, itching) in women with a history of penicillin allergy who are given cephalosporins is 1% to 10%. The incidence of anaphylaxis in this setting is less than 0.02% (72).
A single dose of broad-spectrum antibiotics can result in pseudomembranous colitis, caused by Clostridium difficile. Diarrhea may develop in as many as 15% of hospitalized patients treated with beta-lactam antibiotics (73). In patients receiving clindamycin, the rate of diarrhea is nearly 10% to 25% (74). These gastrointestinal complications from antibiotics may cause serious morbidity in the surgical patient, and the surgeon should be able to recognize and manage these problems.
Not all gynecologic surgery patients need to receive prophylactic antibiotics. The surgeon should choose agents to cover procedures based on available data, thereby avoiding the potential for adverse reactions and minimizing the unnecessary use of antibiotics, which may contribute to increased rates of antimicrobial resistance. In patients with cephalosporin allergies or anaphylaxis to penicillin, other drugs or combinations should be chosen to provide adequate prophylactic coverage. Antimicrobial prophylaxis options for common gynecologic procedures are presented in Table 22.6. Antibiotic prophylaxis is not indicated for diagnostic or operative laparoscopy, exploratory laparotomy, or diagnostic or operative hysteroscopy, including endometrial ablation, intrauterine device insertions, endometrial biopsy, or urodynamics (75).
Subacute Bacterial Endocarditis Prophylaxis
It was thought that women who had severe valvular disease or other cardiac conditions required antibiotic prophylaxis prior to genitourinary (GU) or gastrointestinal (GI) procedures in order to prevent bacterial endocarditis as a result of the transient bacteremia provoked by the surgery. After reviewing the pertinent evidence-based literature, the American Heart Association issued revised guidelines in 2007 stating that antibiotic prophylaxis was not necessary solely to prevent endocarditis in patients undergoing GI or GU procedures, including hysterectomy (Table 22.8) (76).
Table 22.8 Recommendations for Prophylaxis of Bacterial Endocarditis
| Highest-Risk Patients | Agents | Regimen (within 30 – 60 min of starting procedure) |
| Standard regimen | Amoxicillin | 2 g PO |
| Ampicillin | 2.0 g IM or IV | |
| or | ||
| Cefazolin or ceftriaxone | 1 g IM or IV | |
| Cephalexin | 2 g | |
| Penicillin-allergic (oral) | Cephalexin | 2 g |
| Clindamycin | 600 mg | |
| Azithromycin or clarithromycin | 500 mg | |
| Penicillin-allergic (non-oral) | Cefazolin or ceftriaxone | 1 g IM or IV |
| Clindamycin | 600 mg IM or IV | |
| IM, intramuscularly; IV, intravenously. | ||
| Derived from Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2007;116:1736–1754 | ||
Postoperative Infections
Infections are a major source of morbidity in the postoperative period. Risk factors for infectious morbidity include the absence of perioperative antibiotic prophylaxis, contamination of the surgical field from infected tissues or from spillage of large bowel contents, an immunocompromised host, poor nutrition, chronic and debilitating severe illness, poor surgical technique, and preexisting focal or systemic infection. Sources of postoperative infection can include the lung, urinary tract, surgical site, pelvic sidewall, vaginal cuff, abdominal wound, and sites of indwelling intravenous catheters. Early identification and treatment of infection will result in the best outcome for these potentially serious complications.
Although infectious morbidity is an inevitable complication of surgery, the incidence of infections can be decreased by the appropriate use of simple preventive measures. In cases that involve transection of the large bowel, spillage of fecal contents inevitably occurs. A thorough preoperative mechanical and antibiotic bowel preparation in combination with systemic antibiotic prophylaxis will help decrease the incidence of postoperative pelvic and abdominal infections in these patients. The surgeon can further decrease the risk of postoperative infections by using meticulous surgical technique. Blood and necrotic tissue are excellent media for the growth of aerobic and anaerobic organisms. In cases in which there is higher-than-usual potential for serum and blood to collect in spaces that were contaminated by bacterial spill, closed-suction drainage may reduce the risk of infection. Antibiotic therapy, rather than prophylaxis, should be initiated during surgery in patients who have frank intra-abdominal infection or pus. Elective surgical procedures should be postponed in patients who have preoperative infections. In an epidemiologic study conducted by the Centers for Disease Control and Prevention (CDC), the incidence of nosocomial surgical infections ranged from 4.3% in community hospitals to 7% in municipal hospitals (77). Data confirmed this, with an incidence of 2% to 5% (78). Urinary tract infections accounted for approximately 40% of these nosocomial infections. Infections of the skin and wound accounted for approximately one-third of the infections, and respiratory tract infections accounted for approximately 16%. In patients who had any type of infection before surgery, the risk of infection at the surgical wound site increased fourfold. Rates of infection were higher in older patients, in patients with increased length of surgery, and in those with increased length of hospital stay before surgery. The relative risk was three times higher in patients with a community-acquired infection before surgery. These community-acquired infections included infections of the urinary and respiratory tracts.
Historically, the standard definition of febrile morbidity for surgical patients was the presence of a temperature higher than or equal to 100.4°F (38°C) on two occasions at least 4 hours apart during the postoperative period, excluding the first 24 hours. Other sources defined fever as two consecutive temperature elevations greater than 101.0°F (38.3°C) (79,80). Febrile morbidity is estimated to occur in as many as one-half of patients; it is often self-limited, resolves without therapy, and is usually noninfectious in origin (81). Overzealous evaluations of postoperative fever, especially during the early postoperative period, are time consuming, expensive, and sometimes uncomfortable for the patient (81). The value of 101.0°F is more useful than 100.4°F to distinguish an infectious cause from an inconsequential postoperative fever.
The assessment of a febrile surgical patient should include a review of the patient’s history with regard to risk factors. Both the history and the physical examination should focus on the potential sites of infection (Table 22.9). The examination should include inspection of the pharynx, a thorough pulmonary examination, percussion of the kidneys to assess for costovertebral angle tenderness, inspection and palpation of the abdominal incision, examination of sites of intravenous catheters, and an examination of the extremities for evidence of deep venous thrombosis or thrombophlebitis. In gynecologic patients, an appropriate workup may include inspection and palpation of the vaginal cuff for signs of induration, tenderness, or purulent drainage. A pelvic examination should be performed to identify a mass consistent with a pelvic hematoma or abscess and to look for signs of pelvic cellulitis.
Table 22.9 Posthysterectomy Infections
| Operative Site | Nonoperative Site |
| Vaginal cuff | Urinary tract |
| Pelvic cellulitis | Asymptomatic bacteriuria |
| Pelvic abscess | Cystitis |
| Supervaginal, extraperitoneal | Pyelonephritis |
| Intraperitoneal | Respiratory |
| Adnexa | Atelectasis |
| Cellulitis | Pneumonia |
| Abscess | Vascular |
| Abdominal incision | Phlebitis |
| Cellulitis | Septic pelvic thrombophlebitis |
| Simple | |
| Progressive bacterial synergistic | |
| Necrotizing fasciitis | |
| Myonecrosis |
Patients with fever in the early postoperative period should have an aggressive pulmonary toilet, including incentive spirometry (80). If the fever persists beyond 72 hours postoperatively, additional laboratory and radiologic data may be obtained. The evaluation may include complete and differential white blood cell counts and a urinalysis. In one study, results from fever workups included positive blood cultures in 9.7% of patients, a positive urine culture in 18.8%, and a positive chest x-ray in 14%. These data support the need for a tailored workup based on the patient’s clinical picture (82). Blood cultures can be obtained but will most likely be of little yield unless the patient has a high fever (102°F). In patients with costovertebral angle tenderness, intravenous pyelogram may be indicated to rule out the presence of ureteral damage or obstruction from surgery, particularly in the absence of laboratory evidence of urinary tract infection. Patients who have persistent fevers without a clear localizing source should undergo CT scanning of the abdomen and pelvis to rule out the presence of an intra-abdominal abscess. If fever persists in patients who had gastrointestinal surgery, a barium enema or upper gastrointestinal studies with small bowel assessment may be indicated late in the course of the first postoperative week to rule out an anastomotic leak or fistula.
Urinary Tract Infections
Historically, the urinary tract was the most common site of infection in surgical patients (83). The incidence reported in the gynecologic literature is less than 4% (84,85). This decrease in urinary tract infections is most likely the result of increased perioperative use of prophylactic antibiotics. The incidence of postoperative urinary tract infection in gynecologic surgical patients not receiving prophylactic antibiotics is confirmed to be as high as 40%, and even a single dose of perioperative prophylactic antibiotic decreases the incidence of postoperative urinary tract infection to as low as 4% (86,87).
Symptoms of a urinary tract infection may include urinary frequency, urgency, and dysuria. In patients with pyelonephritis, other symptoms include headache, malaise, nausea, and vomiting. A urinary tract infection is diagnosed on the basis of microbiology and is defined as the growth of 105 organisms per milliliter of urine cultured. Most infections are caused by coliform bacteria, with Escherichia coli being the most frequent pathogen. Other pathogens include Klebsiella, Proteus, and Enterobacter species. Staphylococcus organisms are the causative bacteria in fewer than 10% of cases.
Despite the high incidence of urinary tract infections in the postoperative period, few of these infections are serious. Most are confined to the lower urinary tract, and pyelonephritis is a rare complication (88). Catheterization of the urinary tract, either intermittently or continuously with the use of an indwelling catheter, is implicated as a main cause of urinary tract contamination (89). More than 1 million catheter-associated urinary tract infections occur yearly in the United States, and catheter-associated bacteria remains the most common etiology of gram-negative bacteremia in hospitalized patients. Bacteria adhere to the surface of urinary catheters and grow within bile films, which appear to protect embedded bacteria from antibiotics, making treatment less effective. The use of urinary tract catheters should be minimized. An indwelling catheter should be removed or replaced in a patient undergoing treatment for catheter-related infections.
The treatment of urinary tract infection includes hydration and antibiotic therapy. Commonly prescribed and effective antibiotics include penicillin, sulfonamides, cephalosporins, fluoroquinolones, and nitrofurantoin. The choice of antibiotic should be based on knowledge of the susceptibility of organisms cultured at a particular institution. In some institutions, for example, more than 40% of E. coli strains are resistant to ampicillin. For uncomplicated urinary tract infections, an antibiotic that has good activity against E. coli should be given in the interim while awaiting results of the urine culture and sensitivity data.
Patients who have a history of recurrent urinary tract infections, those with chronic indwelling catheters (Foley catheters or ureteral stents), and those who have urinary conduits should be treated with antibiotics that will be effective against the less common urinary pathogens such as Klebsiella and Pseudomonas. Chronic use of fluoroquinolones for prophylaxis is not advised because these agents are notorious for inducing antibiotic-resistant strains of bacteria.
Pulmonary Infections
The respiratory tract is an uncommon site for infectious complications in gynecologic surgical patients. In one study only six cases of pneumonia occurred in more than 4,000 women who underwent elective hysterectomy (85). This low incidence is probably a reflection of the young age and good health status of gynecologic patients in general. In acute care facilities, pneumonia is a frequent hospital-acquired infection, particularly in elderly patients (90). Risk factors include extensive or prolonged atelectasis, preexistent COPD, severe or debilitating illness, central neurologic disease causing an inability to clear oropharyngeal secretions effectively, nasogastric suction, and a prior history of pneumonia (90,91). In surgical patients, early ambulation and aggressive management of atelectasis are the most important preventive measures. The role of prophylactic antibiotics remains unclear.
A significant percentage (40% to 50%) of cases of hospital-acquired pneumonia is caused by gram-negative organisms (83). These organisms gain access to the respiratory tract from the oral pharynx. Gram-negative colonization of the oral pharynx is increased in patients in acute care facilities and is associated with the presence of nasogastric tubes, preexisting respiratory disease, mechanical ventilation, and tracheal intubation (92). The use of antimicrobial drugs seems to significantly increase the frequency of colonization of the oral pharynx with gram-negative bacteria.
A thorough lung examination should be included in the assessment of all febrile surgical patients. In the absence of significant lung findings, chest radiography is probably of little benefit in patients at low risk for postoperative pulmonary complications. In patients with pulmonary findings or with risk factors for pulmonary complications, chest radiography should be performed. A sputum sample should be obtained for Gram stain and culture. The treatment should include postural drainage, aggressive pulmonary toilet, and antibiotics. The antibiotic chosen should be effective against both gram-positive and gram-negative organisms. In patients who are receiving assisted ventilation, the antibiotic spectrum should include drugs that are active against Pseudomonas organisms.
Phlebitis
Historically, intravenous catheter–related infections were common; the reported incidence is 25% to 35% in the 1980s (93). Because the incidence of catheter-related phlebitis increases significantly after 72 hours, intravenous catheters should be changed at least every 3 days according to the CDC (94). The institution of intravenous therapy teams decreased the incidence of phlebitis by as much as 50% in one study (95). In combination, these measures led to a dramatic decrease in peripheral catheter site infection.
The intravenous site should be inspected daily, and the catheter should be removed if there is any associated pain, redness, or induration. Phlebitis can occur even with close surveillance of the intravenous site. In one study, more than 50% of the cases of phlebitis became evident more than 12 hours after discontinuation of intravenous catheters (96). Less than one-third of patients had symptoms related to the intravenous catheter site 24 hours before the diagnosis of phlebitis.
Phlebitis can be diagnosed based on the presence of fever, pain, redness, induration, or a palpable venous cord. Occasionally, suppuration will be present. Phlebitis is usually self-limited and resolves within 3 to 4 days. The treatment includes application of warm, moist compresses and prompt removal of any catheters from the infected vein. Antibiotic therapy with antistaphylococcal agents should be instituted for catheter-related sepsis. Excision or drainage of an infected vein rarely is necessary.
Wound Infections
The results of a prospective study of more than 62,000 wounds were revealing in regard to the epidemiology of wound infections (97). The wound infection rate varied markedly, depending on the extent of contamination of the surgical field. The wound infection rate for clean surgical cases (infection not present in the surgical field, no break in aseptic technique, no viscus entered) was lower than 2%, whereas the incidence of wound infections with dirty, infected cases was 40% or higher. Preoperative showers with hexachlorophene slightly lowered the infection rate for clean wounds, whereas preoperative shaving of the wound site with a razor increased the infection rate. A 5-minute wound preparation immediately before surgery was as effective as preparation for 10 minutes. The wound infection rate increased with the duration of preoperative hospital stay and with the duration of surgery. Incidental appendectomy increased the risk of wound infection in patients undergoing clean surgical procedures. The study concluded that the incidence of wound infections could be decreased by short preoperative hospital stays, hexachlorophene showers before surgery, minimizing shaving of the wound site, use of meticulous surgical technique, decreasing operative time as much as possible, bringing drains out through sites other than the wound, and dissemination of information to surgeons regarding their wound infection rates. A program instituting these conclusions led to a decrease in the clean wound infection rate from 2.5% to 0.6% over an 8-year period. The wound infection rate in most gynecologic services is lower than 5%, reflective of the clean nature of most gynecologic operations.
The symptoms of wound infection often occur late in the postoperative period, usually after the fourth postoperative day, and may include fever, erythema, tenderness, induration, and purulent drainage. Wound infections that occur on postoperative days 1 through 3 are generally caused by streptococcal and Clostridia infections. The management of wound infections is mostly mechanical and involves opening the infected portion of the wound above the fascia, with cleansing and debridement of the wound edges as necessary. Wound care, consisting of debridement and dressing changes two to three times daily with mesh gauze, will promote growth of granulation tissue, with gradual filling in of the wound defect by secondary intention. Clean, granulating wounds can often be secondarily closed with good success, shortening the time required for complete wound healing.
The technique of delayed primary wound closure can be used in contaminated surgical cases to lower the incidence of wound infection. This technique involves leaving the wound open above the fascia at the time of the initial surgical procedure. Vertical interrupted mattress sutures through the skin and subcutaneous layers are placed 3 cm apart but are not tied. Wound care is instituted immediately after surgery and continued until the wound is noted to be granulating well. Sutures may then be tied and the skin edges further approximated using sutures or staples. Using this technique of delayed primary wound closure, the overall wound infection rate is decreased from 23% to 2.1% in high-risk patients (98).
Pelvic Cellulitis
Vaginal cuff cellulitis is present in most patients who underwent hysterectomy. It is characterized by erythema, induration, and tenderness at the vaginal cuff. A purulent discharge from the apex of the vagina may be present. The cellulitis is often self-limited and does not require any treatment. Fever, leukocytosis, and pain localized to the pelvis may accompany severe cuff cellulitis and most often signifies extension of the cellulitis to adjacent pelvic tissues. In such cases, broad-spectrum antibiotic therapy should be instituted with coverage for gram-negative, gram-positive, and anaerobic organisms. If purulence at the vaginal cuff is excessive or if there is a fluctuant mass noted at the vaginal cuff, the vaginal cuff should be gently probed and opened with a blunt instrument. The cuff can be left open for dependent drainage or, alternatively, a drain can be placed into the lower pelvis through the cuff and removed when drainage, fever, and symptoms in the lower pelvic region have resolved.
Intra-abdominal and Pelvic Abscess
The development of an abscess in the surgical field or elsewhere in the abdominal cavity is an uncommon complication after a gynecologic surgery. It is likely to occur in contaminated cases in which the surgical site is not adequately drained or as a secondary complication of hematomas. The causative pathogens in patients who have intra-abdominal abscesses are usually polymicrobial in nature. The aerobes most commonly identified include E. coli, Klebsiella, Streptococcus, Proteus, and Enterobacter. Anaerobic isolates are common, usually from the Bacteroides group. These pathogens arise mainly from the vaginal tract but can be derived from the gastrointestinal tract, particularly when the colon was entered at the time of surgery.
Intra-abdominal abscess is sometimes difficult to diagnose. The evolving clinical picture is often one of persistent febrile episodes with a rising white blood cell count. Findings on abdominal examination may be equivocal. If an abscess is located deep in the pelvis, it may be palpable by pelvic or rectal examination. For abscesses above the pelvis, the diagnosis will depend on radiologic confirmation.
Ultrasonography can occasionally delineate fluid collections in the upper abdomen and in the pelvis. Bowel gas interference makes visualization of fluid collections or abscesses in the midabdomen difficult to distinguish. Computed tomography scanning is more sensitive and specific for diagnosing intra-abdominal abscesses and often is the radiologic procedure of choice. Occasionally, if conventional radiologic methods fail to identify an abscess and the index of suspicion for an abscess remains high, labeled leukocyte scanning may be useful for locating the infected focus.
Standard therapy for intra-abdominal abscess is evacuation and drainage combined with appropriate parenteral administration of antibiotics. Abscesses located low in the pelvis, particularly in the area of the vaginal cuff, can be reached through a vaginal approach. In many patients, the ability to drain an abscess by placement of a drain percutaneously under CT guidance obviated the need for surgical exploration. With CT guidance, a pigtail catheter is placed into an abscess cavity via transperineal, transrectal, or transvaginal approaches. The catheter is left in place until drainage decreases. Transperineal and transrectal drainage of deep pelvic abscesses is successful in 90% to 93% of patients, obviating the need for surgical management (99,100). For those patients in whom radiologic drainage is not successful, surgical exploration and evacuation are indicated. The standard approach to initial antibiotic therapy is the combination of ampicillin, gentamicin, and clindamycin. Adequate treatment can be achieved with available broad-spectrum single agents (including the broad-spectrum penicillin), second- and third-generation cephalosporins, levofloxacin and metronidazole, and the sulbactam-clavulanic acid–containing preparations (101).
Necrotizing Fasciitis
Necrotizing fasciitis is an uncommon infectious disorder, affecting roughly 1,000 patients per year (102). This disease process is characterized by a rapidly progressive bacterial infection that involves the subcutaneous tissues and fascia while characteristically sparing underlying muscle. Systemic toxicity is a frequent feature of this disease, as manifested by the presence of dehydration, septic shock, disseminated intravascular coagulation, and multiple organ system failure.
The pathogenesis of necrotizing fasciitis involves a polymicrobial infection of the dermis and subcutaneous tissue. Hemolytic streptococcus was initially believed to be the primary pathogen responsible for the infection in necrotizing fasciitis (103). Other organisms are often cultured in addition to streptococcus, including other gram-positive organisms, coliforms, and anaerobes (104). Bacterial enzymes such as hyaluronidase and lipase released in the subcutaneous space destroy the fascia and adipose tissue and induce a liquefactive necrosis. Noninflammatory intravascular coagulation or thrombosis subsequently occurs. Intravascular coagulation results in ischemia and necrosis of the subcutaneous tissues and skin. Subcutaneous spread of up to 1 inch per hour can be seen, often with little effect on the overlying skin (104). Late in the course of the infection, destruction of the superficial nerves produces anesthesia in the involved skin. The release of bacteria and bacterial toxins into the systemic circulation can cause septic shock, acid-base disturbances, and multiple organ impairment.
The diagnosis is often difficult to make early in the disease course. Most patients with necrotizing fasciitis develop erythema, edema, and pain, which in the early stages of the disease is disproportionately greater than that expected from the degree of cellulitis present and characteristically extends beyond the border of erythema (105). Late in the course of the infection, the involved skin may be anesthetized secondary to necrosis of superficial nerves. Temperature abnormalities, both hyperthermia and hypothermia, are concomitant with the release of bacterial toxins and with bacterial sepsis (104). The involved skin is initially tender, erythematous, and warm. Edema develops, and the erythema spreads diffusely, fading into normal skin, characteristically without distinct margins or induration. Subcutaneous microvascular thrombosis induces ischemia in the skin, which becomes cyanotic and blistered. As necrosis develops, the skin becomes gangrenous and may slough spontaneously (104). Most patients will have leukocytosis and acid-base abnormalities. Subcutaneous gas may develop, which can be identified by palpation and by radiography. The finding of subcutaneous gas by radiography is often indicative of clostridial infection, although it is not a specific finding and may be caused by other organisms. These organisms include Enterobacter, Pseudomonas, anaerobic streptococci, and Bacteroides, which, unlike clostridial infections, spare the muscles underlying the affected area. A tissue biopsy specimen for Gram stain and aerobic and anaerobic culture should be obtained from the necrotic center of the lesion to identify the etiologic organisms (105). Although necrotizing fasciitis often is diagnosed during surgery, a high index of suspicion and liberal use of frozen-section biopsy can provide an early life-saving diagnosis and minimize morbidity (104).
Predisposing risk factors for necrotizing fasciitis include diabetes mellitus, alcoholism, an immunocompromised state, hypertension, peripheral vascular disease, intravenous drug abuse, and obesity (104). The most frequent site of infection is in the extremities, but the infection can occur anywhere in the subcutaneous tissues, including the head and neck, trunk, and perineum. Necrotizing fasciitis occurs after trauma, surgery, burns, and lacerations; as a secondary complication in perirectal infections or Bartholin duct abscesses; and de novo (103,106–109). Increased age, delay in diagnosis, inadequate debridement during initial surgery, extensive disease at the time of diagnosis, and the presence of diabetes mellitus are all factors that are associated with an increased likelihood of mortality from necrotizing fasciitis (104–106). Early diagnosis and aggressive management of this lethal disease contribute to improved survival.
Successful management of necrotizing fasciitis involves early recognition, immediate initiation of resuscitative measures (including correction of fluid, acid-base, electrolyte, and hematologic abnormalities), aggressive surgical debridement and redebridement as necessary, and broad-spectrum intravenous antibiotic therapy (104). During surgery, the incision should be made through the infected tissue down to the fascia. An ability to undermine the skin and subcutaneous tissues with digital palpation often will confirm the diagnosis. Multiple incisions can be made sequentially toward the periphery of the affected tissue until well-vascularized, healthy, resistant tissue is reached at all margins. The remaining affected tissue must be excised. The wound can be packed and sequentially debrided on a daily basis as necessary until healthy tissue is displayed at all margins. Hyperbaric oxygen therapy may be of some benefit, particularly in patients for whom culture results are positive for anaerobic organisms (110). Retrospective nonrandomized studies demonstrated that the addition of hyperbaric oxygen therapy to surgical debridement and antimicrobial therapy appears to significantly decrease both wound morbidity and overall mortality in patients with necrotizing fasciitis (110). The benefit of hyperbaric therapy demonstrated in one study was remarkable, given that patients receiving hyperbaric oxygen were sicker and had a higher incidence of diabetes mellitus, leukocytosis, and shock (106).
After the initial resuscitative efforts and surgical debridement, the primary concern is the management of the open wound. Allograft and xenograft skin can be used to cover open wounds, thus decreasing heat and evaporative water loss. Temporary biologic closure of open wounds seems to decrease bacterial growth (111). Amniotic membranes were effective wound covering in patients with necrotizing fasciitis (112).
A new technology demonstrated to significantly improve wound healing in laboratory and clinical studies is a vacuum-assisted closure (VAC) method that uses a subatmospheric pressure technique (113–115). In situations in which spontaneous closure is not likely, the VAC device may allow for the development of a suitable granulation bed and prepare the tissue for graft placement, thereby increasing the probability of graft survival. Skin flaps can be mobilized to help cover open wounds when the infections resolve and granulation begins.
Gastrointestinal Preparation
Traditionally, mechanical bowel preparation was advised before abdominal surgery, especially if colonic surgery was anticipated. Despite the infrequent need for colonic surgery (or injury) when performing gynecologic surgery, bowel preparation is part of the standard practice for many gynecologists. Advantages of mechanical bowel preparation include reduction of gastrointestinal contents, which facilitates the surgical procedure by allowing more room in the abdomen and pelvis. If a rectosigmoid colon enterotomy occurs, the mechanical bowel preparation eliminates formed stool and reduces the risk of bacterial contamination, thus reducing infectious complications.
Randomized clinical trials questioned the need for mechanical bowel preparation in colonic surgery (116). Although somewhat controversial, a meta-analyses including almost 5,000 patients showed no statistical difference between the groups for anastomotic leakage (p = 0.46), pelvic or abdominal abscess (p = 0.75), and wound sepsis (p = 0.11). The use of different mechanical regimes did not influence primary and secondary outcomes. The authors concluded that this analysis demonstrates that any kind of mechanical bowel preparation should be omitted before colonic surgery. The main limitation concerned rectal surgery for which the limited data preclude any interpretation.
A randomized trial of mechanical bowel preparation versus no bowel preparation with rectal surgery found that the overall and site-specific infectious morbidity rates were significantly higher in no preparation versus the mechanical preparation group. Regarding anastomotic leakage, length of hospital stay, major morbidity and mortality rates, there was no significant difference between the no preparation and mechanical bowel preparation groups. This was the first randomized trial to demonstrate that rectal cancer surgery without mechanical preparation is associated with higher risk of overall and infectious morbidity rates without any significant increase of anastomotic leakage rate. It suggests continuing to perform mechanical preparation before elective rectal resection for cancer (117).
Despite evidence of the infectious complications associated with mechanical preparation or no preparation, many gynecologic surgeons prefer a mechanical preparation to aid with exposure in the pelvic operative field. This may be particularly relevant to the surgeon performing minimally invasive surgery.
Mechanical bowel preparation may be accomplished as presented in (Table 22.10) The traditional use of laxatives and enemas requires at least 12 to 24 hours and causes moderate abdominal distention and crampy pain. Randomized trials comparing traditional mechanical bowel preparation (magnesium citrate and enemas) with oral gut lavage (PEG electrolyte solution, GoLYTELY) found that the use of approximately 4 L of GoLYTELY (administered until the rectal effluent is clear) provides more complete, faster, and more comfortable bowel preparation (118). However, the ingestion of 4 L of fluid is problematic for many patients, so magnesium citrate may be preferable when mechanical preparation is to be used.
Table 22.10 Mechanical Bowel Preparation
| Preoperative Day 1 Clear liquid diet |
| Mechanical Prep |
| 4 L of GoLYTELY OR Fleet Phospho-Soda Saline laxative (3 oz. bottle)a (one bottle of laxative 1 p.m. and another at 7 p.m.) Fleet enemas until no solid stool in p.m. (optional) |
| Antibiotic Prep (Optional) |
| Neomycin (oral), 1 g every 4 h for three doses (4, 8, 12 p.m.) Erythromycin (oral), 1 g every 4 h for three doses or metronidazole 500 mgm every 4 h for three doses |
aBe aware of acute phosphate nephropathy. Avoid use of phospho-soda in patients with inadequate hydration, increased age, a history of hypertension, current use of an angiotensin receptor blocker, or angiotensin-conversing enzyme (ACE) inhibitors; renal disease or chronic heart failure and those taking nonsteroidal anti-inflammatory drugs (NSAIDs) or diuretics. |
An alternative mechanical preparation method is the use of oral sodium phosphate (Phospho-Soda). When evaluated in a randomized trial comparing the 4 L of GoLYTELY with oral sodium phosphate, colonoscopic examination disclosed that both methods were equally effective in colonic cleansing, and more patients preferred the sodium phosphate method (119). Some patients are at higher risk to develop acute phosphate nephropathy, leading to acute renal failure or worsening of chronic renal disease after the use of sodium phosphate. Although the pathophysiology is not fully understood, it may be secondary to substantial fluid shifts and electrolyte changes. It is speculated that potential etiologic factors included inadequate hydration, increased patient age, a history of hypertension, and current use of an angiotensin receptor blocker or angiotension-converting enzyme (ACE) inhibitors. Patients with chronic renal disease or chronic heart failure and those taking NSAIDs or diuretics appear to be at higher risk of acute phosphate nephropathy (120). For these patients, the guidelines recommend an alternative bowel preparation agent, polyethylene glycol, which is not associated with volume shifts and electrolyte abnormalities (121,122).
Oral antibiotic prophylaxis was advised over the past three decades to reduce the infectious complications following colonic surgery. The usual regimen was a combination of oral erythromycin and neomycin taken the day before surgery. Many surgeons would substitute oral metronidazole for erythromycin because patients tolerate it better. With the use of perioperative parental antibiotics, the benefit of oral antibiotics is questioned and most surgeons abandoned the use of oral antibiotics in favor of perioperative parenteral antibiotics.
Postoperative Gastrointestinal Complications
Ileus
Following open abdominal or pelvic surgery, most patients experience some degree of intestinal ileus. The exact mechanism by which this arrest and disorganization of gastrointestinal motility occurs is unknown, but it appears to be associated with the opening of the peritoneal cavity and is aggravated by manipulation of the intestinal tract and prolonged surgical procedures. Infection, peritonitis, and electrolyte disturbances may result in ileus. For most patients undergoing common gynecologic operations, the degree of ileus is minimal, and gastrointestinal function returns relatively rapidly, allowing the resumption of oral intake within a few days of surgery. Patients who have persistently diminished bowel sounds, abdominal distention, and nausea and vomiting require further evaluation and more aggressive management. Patients with symptoms of ileus or small bowel obstruction who underwent minimally invasive surgery are a different matter. Minimally invasive surgery should result in a daily improvement in GI function. An “ileus” in the case of minimally invasive surgery more likely represents GI injury, which should be evaluated immediately with a CT scan using GI contrast.
Ileus is usually manifested by abdominal distention and should be evaluated by physical examination. Pertinent points of the abdominal examination include assessment of the quality of bowel sounds and palpation in search of distension, masses, tenderness, or rebound. The possibility that the patient’s signs and symptoms may be associated with a more serious intestinal obstruction or intestinal complication (such as a perforation) must be considered. Pelvic examination should be performed to evaluate the possibility of a pelvic abscess or hematoma that may contribute to the ileus. Abdominal radiography to evaluate the abdomen in the flat (supine) position and in the upright position will aid in the diagnosis of an ileus. The most common radiographic findings include dilated loops of small and large bowel and air-fluid levels while the patient is in the upright position. Sometimes, massive dilation of the colon or stomach may be noted. The remote possibility of distal colonic obstruction suggested by a dilated cecum should be excluded by rectal examination, proctosigmoidoscopy, colonoscopy, or barium enema. In the postoperative gynecology patient, especially in the upright position, the abdominal x-ray may show free air. This common finding following surgery lasts 7 to 10 days in some instances and is not indicative of a perforated viscus in most patients.
The initial management of a postoperative ileus is aimed at gastrointestinal tract decompression and maintenance of appropriate intravenous replacement fluids and electrolytes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


