Pathological triggers of preterm birth.
Infection plays a role in ~30–40% of spontaneous preterm births. The commonest route of infection is the ascent of pathogens, through the cervix, from the lower genital tract. Haematogenous spread via the placenta is also recognized (as illustrated by studies revealing colonization of the amniotic cavity with gingival microorganisms). Infection may be limited to the choriodecidual interface, or may spread across the membranes causing chorioamnionitis or even fetal infection (with a resultant fetal inflammatory response syndrome, FIRS). Microorganisms present within the uterus can stimulate contractions through the release of phospholipase A, which provokes local prostaglandin production. They also stimulate cervical ripening and weakening of the fetal membranes through the production of matrix metalloproteases/other proteolytic enzymes and heighten inflammatory mediator release (particularly interleukin-1 and tumour necrosis factor), which further drives prostaglandin production.
Over-distension of the uterus (as seen in multiple pregnancy, polyhydramnios or mullerian abnormalities) has been shown to induce increased expression of so-called ‘contraction-associated proteins’. Such proteins include the oxytocin receptor, prostaglandin synthetic enzymes and receptors, and gap junction proteins (e.g. connexions) which facilitate propagation of action potentials and thus muscular contraction throughout the uterus.
Uteroplacental ischaemia (both chronic, as seen in intrauterine growth restriction, and acute, as seen in placental abruption) seems to provoke early labour. Observational studies have noted an increased incidence of abnormal vasculature in the placentas of preterm babies, such as failed transformation of the spiral arteries and thrombotic lesions. The mechanisms by which ischaemia precipitates labour are unclear, but stimulation of the local renin–angiotensin–aldosterone system or direct stimulation of uterine contractility by thrombin and hypoxia may play a role.
Cervical weakness is a well-recognized contributor to preterm birth. Women may have inherent problems with cervical function, or insufficiency may follow cervical surgery (particularly cone biopsy or multiple loop excisions). An overlap between infection-mediated preterm birth and cervical shortening has also become increasingly clear. Women with a short cervix have a much higher incidence of intrauterine infection. However, in practice it is unclear whether this finding is explained by reduced barrier function of the shortened cervix (which increases the risk of ascending infection) or by the reverse scenario, where intrauterine infection stimulates cervical ripening and shortening.
Understanding the causes and underlying pathways of preterm labour is key to developing effective tests and treatment, and knowledge in this area is continually developing.
Antenatal management
Strategies aiming to reduce the morbidity and mortality associated with preterm birth require the following steps:
identification of women at increased risk of preterm birth
provision of surveillance/screening tests
administration of preventive treatments
careful assessment and treatment of those presenting with threatened preterm labour
Risk assessment
A multitude of factors have been associated with increased preterm birth (PTB) risk, some of which are modifiable, others not. Table 5.1 provides a summary of risk factors; this list is not exhaustive, but the presence of these features in a woman’s history may be helpful in assessing and reducing her risk in future pregnancies.
| Obstetric history | History of previous preterm birth/late miscarriage History of previous preterm premature rupture of membranes (PPROM) Prior obstetric cervical injury (e.g. tearing during vaginal delivery or second-stage caesarean section) |
| Maternal demographics | Extremes of maternal age Ethnicity (higher rates in non-white, particularly black, mothers) Socioeconomic status* Poor access to antenatal care* |
| Gynaecological history | Cervical treatment (especially previous cone biopsy/multiple loop excisions) Known bicornuate uterus/other mullerian abnormality Uterine septoplasty |
| Lifestyle factors | Smoking* Stress* Activity levels* (shift work/heavy physical labour associated with PTB) BMI* Short inter-pregnancy interval* Periodontitis* |
| Pregnancy-specific factors | Bleeding/antepartum haemorrhage Short cervix (< 25 mm before 24 weeks) Bacterial vaginosis (BV) Multiple pregnancy* Fertility treatment |
* potentially modifiable factors
A history of a previous preterm birth is the strongest historical predictor of early delivery. The recurrence rate for PTB in a singleton pregnancy following prior singleton PTB is ~20–25%. However, recurrence rates vary according to gestation of prior PTB (the earlier the gestation the higher the recurrence risk), number of prior preterm births and the occurrence of any term deliveries following the index PTB. Careful history taking is essential to assess the nature of the prior PTB and whether any non-recurrent triggers were present (e.g. traumatic abruption), to allow individualized counselling of patients.
Many of the other factors detailed above lack sensitivity in predicting preterm birth recurrence, but identifying modifiable risk factors will enable the woman to make changes to reduce her risk (including weight reduction, smoking cessation and modification of working patterns and life stressors). For healthcare providers, ensuring good access to antenatal care, particularly for vulnerable women, maximizing the availability of antenatal monitoring and avoiding iatrogenic multiple pregnancy (through cautious use of reproductive technologies) will all help reduce the preterm birth rate.
Investigations
Cervical length scanning
Ultrasound measurement of cervical length has an established role in preterm birth prediction. Although the cervix can be assessed by transabdominal and translabial routes, transvaginal ultrasound most sensitively identifies cervical shortening, is generally acceptable to women and has good reproducibility when performed by trained practitioners (Figure 5.2).
Transvaginal ultrasound illustrating (a) a normal cervical length and (b) a shortened cervix demonstrating funnelling.
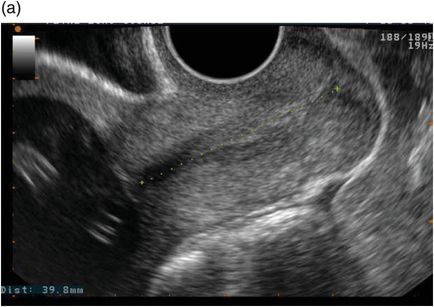
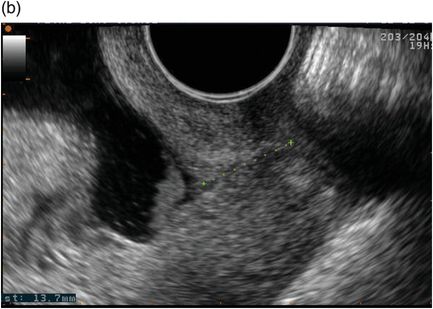
The technique for measuring cervical length is relatively simple:
Ask the woman to empty her bladder.
Gently introduce the covered transvaginal probe, while observing the image on screen and taking care not to compress the cervix excessively.
Obtain a longitudinal section of the uterus and cervix, noting the presence of any funnelling or ‘sludge’ at the level of the internal os.
Magnify the image to occupy two-thirds of the screen.
Once a suitable image demonstrating the full length of the cervix has been obtained, withdraw the transducer slightly, enough to minimize application pressure to the cervix but maintain image quality.
Place the calipers on the internal and external os and measure the cervical length (if the cervix is excessively curved a linear measurement may underestimate this).
Repeat the measurement, obtaining three images with good reproducibility, and record the shortest obtained cervical length.
Several practice guidelines recommend that all women with a history of one or more prior preterm births or mid-trimester losses are offered serial cervical length scans, but acknowledge that expectant management may also be appropriate, as the majority of women with a history of preterm birth will go on to deliver after more than 33 weeks gestation. Screening regimes vary, but commonly scans are performed fortnightly, between 16 and 24 weeks. A cervical length ≥ 25 mm prior to 24 weeks is considered normal for women with a prior preterm birth, although thresholds for initiating treatment vary between studies.
It has been suggested that universal cervical length screening at the time of the anomaly scan might be utilized in the general obstetric population to identify those at high risk of preterm birth. This approach is not without problems: current evidence does not support the use of cervical cerclage in low-risk women with an incidental short cervix, and progesterone use in this group is also contentious. While it has been suggested by some that progesterone supplementation in women with cervical length < 15 mm may be of benefit, such treatment is unlicensed in the UK, and the Royal College of Obstetricians and Gynaecologists (RCOG) recommends restricting its use to clinical trials pending additional evidence. The limited positive predictive value of a short cervix in women without prior preterm birth is also a problem, with estimates ranging widely from 4% to 44%.1
Infection screening
An association between bacterial vaginosis (BV) and preterm birth has long been recognized. The most recent Cochrane review of antibiotic therapy for BV in pregnancy suggests that early screening and treatment can reduce the risk of late miscarriage (RR 0.2, 95% CI 0.05–0.76), but does not seem to reduce the rate of preterm birth < 37 weeks or PPROM.2 Even when women with a prior history of preterm birth were considered, antibiotic treatment did not significantly reduce preterm birth rates. Other studies have suggested that gene–environment interactions may underlie the association between BV and early delivery: researchers have identified polymorphisms within several inflammation-regulating genes which, when present in association with BV, are associated with a particular increase in preterm birth risk. In future, if women with genetic susceptibility to the effects of BV can be identified reliably, they may be a group who demonstrate greater benefit from infection screening.
One large randomized study evaluated in a 2015 Cochrane review suggests that broader screening for lower genital tract infections (obtaining vaginal swabs for BV, candida and trichomonas and treating if positive) may reduce preterm birth (RR 0.55, 95% CI 0.41–0.75), although caregivers were not blinded to the woman’s group assignment or screening results, and it was unclear if loss to follow-up was balanced between groups.3
Screening and treatment for asymptomatic bacteriuria in pregnancy has also been the subject of a systematic review. Its incidence varies from 2% to 10%, and treatment is known to reduce the risk of maternal pyelonephritis (RR 0.23, 95% CI 0.13–0.41). Although antibiotics did not seem to reduce the risk of preterm birth, a lower incidence of low fetal birth weight was noted in mothers receiving screening and treatment.
Periodontitis has also been associated with increased preterm birth rates in observational research. However, studies evaluating the effect of dental treatment on preterm birth rates have yet to show evidence of benefit.
Fetal fibronectin (FFN)
Fetal fibronectin is a glycoprotein which is normally present within the uterus, particularly at the interface between the decidua and membranes. It plays a role in adhesion of the membranes to the uterine wall, and disruption at this level (e.g. due to inflammation or membrane separation) can cause FFN to be released into the cervicovaginal discharge. In normal pregnancies, levels of FFN within the discharge should be low between 20 and 35 weeks gestation; if a high level is detected, this is associated with an increased risk of preterm birth. Current commercial testing kits require a swab to be obtained from the posterior vaginal fornix. This is then placed in a buffer solution and tested with a rapid bedside enzyme-linked immunosorbent assay (ELISA) which utilizes FDC-6 (a monoclonal antibody specific for FFN) to estimate FFN levels within the sample.
Many studies of FFN testing have focused on its use in women with symptoms of preterm labour (considered further below).4 However, it is increasingly being used in the assessment of asymptomatic women at high risk of preterm birth. Testing may be qualitative (< 50 ng/mL represents a negative FFN swab, ≥ 50 ng/mL is a positive result) or quantitative. The most clinically useful aspect of the test is its high negative predictive value (NPV): in asymptomatic high-risk women, NPVs of 86–98% have been reported, with higher NPV when predicting PTB over shorter time periods. Initial studies of quantitative FFN testing suggest improved positive predictive values (PPV): one series suggested a PPV of 62.5% for preterm birth < 37 weeks when FFN levels of ≥ 200 ng/mL were detected in asymptomatic high-risk women. Practically speaking, a negative FFN swab in a patient at high risk of preterm birth may aid decision making (e.g. when deciding if admission is required when cervical shortening has been identified).
Other technologies
A multitude of other biomarkers and imaging techniques continue to be assessed in the hunt for better predictive tests. In a recent systematic review, 116 different serum or cervicovaginal biomarkers were identified but none has yet improved upon tests used in current clinical practice.5 Cervical assessment has also been the focus of considerable work, with novel technologies (e.g. cervical elastography and fluoroscopy) aiming to quantify ripening changes within cervical stroma and the mechanical properties of the tissue in addition to measuring length alone. In future, successful screening may need to incorporate several of these tests, to allow a more comprehensive risk assessment.
In summary, the most successful screening approaches currently focus on monitoring women with a history suggestive of high preterm birth risk. Effective tests and interventions for low-risk populations have not yet been established. In women with risk factors for preterm birth, cervical length surveillance and early swabs for bacterial vaginosis may be offered. Quantitative fibronectin estimation may be offered via specialist clinics, and can aid clinical decision making.
Preventive treatment
Cerclage
Cervical cerclage has been performed for over 100 years, but enthusiasm for the procedure has varied, as conflicting results of trials and meta-analyses have been published. It is unclear whether the inconsistent evidence of benefit in the literature results from inherent limitations of the procedure, or from difficulties selecting the patients who are most likely to benefit. Similarly, it can be difficult to evaluate which complications/side effects of cerclage result from treatment and which are due to underlying cervical incompetence/predisposition to infection. Its utility is further detailed in an RCOG guideline.6
Sutures can be inserted via transvaginal or transabdominal approaches. The transvaginal McDonald technique involves cerclage insertion just below the maternal bladder, and it can be removed without regional anaesthesia. The Shirodkar technique necessitates reflection of the bladder, enabling higher stitch placement, but as a result removal is more difficult. Transabdominal sutures may be placed using open or laparoscopic techniques, and are generally considered in those for whom transvaginal cerclage has failed, or after trachelectomy for neoplastic disease. Ongoing studies are addressing technique-specific questions; at present there are no recommendations regarding choice of suture material, placement of single versus double sutures, etc.
There are three scenarios in which cervical cerclage may be indicated:
in a patient presenting with a history of prior PTB/mid-trimester loss (MTL) (history-indicated cerclage) – prophylactic insertion of a stitch in asymptomatic women at ~12–14 weeks
in a patient with cervical length < 25 mm on transvaginal ultrasound scan (TVUSS) (ultrasound-indicated cerclage) – prophylactic insertion in asymptomatic women with a short cervix on TVUSS at 16–24 weeks
in a patient presenting with premature cervical dilatation (rescue cerclage) – suture insertion once premature cervical dilatation has already commenced
History-indicated cerclage should be offered to women who have experienced three or more previous PTB/MTL. This recommendation is based on the results of a multicentre randomized controlled trial (RCT) which demonstrated a significant reduction in preterm birth rates after cerclage in this group (RR 0.47, p < 0.05), but only non-significant reductions in women with one or two prior PTB/MTL. Two smaller RCTs evaluating history-indicated cerclage in moderate- and high-risk women demonstrated no difference between intervention and control arms. Current UK guidance therefore advises that women with ≤ 2 prior PTB/MTL should be offered ultrasound surveillance of cervical length rather than history-indicated cerclage. A subsequent Cochrane review added weight to this conservative policy: meta-analysis of four studies (of 2045 women at high risk of PTB undergoing history-indicated cerclage or expectant management) showed a non-significant reduction in preterm birth < 37 weeks (RR 0.86, 95% CI 0.59–1.27), and similarly a non-significant reduction in perinatal loss (RR 0.8, 95% CI 0.58–1.1).7 However, patient choice is an important factor in management decisions.
Two meta-analyses have evaluated the use of ultrasound-indicated cerclage versus expectant management in high-risk women with a short cervix. Berghella et al. observed significant reductions in preterm birth < 35 weeks when women with a prior PTB and cervical length < 25 mm were treated (RR 0.70, 95% CI 0.55–0.89).8 They also noted a concurrent reduction in composite perinatal morbidity and mortality (RR 0.64, 95% CI 0.45–0.91). The 2012 Cochrane review by Alfirevic et al. similarly concluded that ultrasound-indicated cerclage can reduce preterm birth < 37 weeks (RR 0.55 [0.30, 0.99] for one-off and 0.78 [0.60, 1.02] for serial ultrasound-indicated cerclage) but did not demonstrate significant reductions in perinatal loss or morbidity.7 UK guidance recommends offering ultrasound-indicated cerclage to high-risk women with cervical length ≤ 25 mm before 24 weeks, but not to women with isolated funnelling, or to low-risk women with an incidental short cervix.
The evidence base for rescue cerclage is very limited, with a few small studies suggesting insertion may delay delivery and reduce preterm birth. However, there is insufficient evidence to confirm whether these gestational gains translate into improved neonatal outcomes. Potential harm, particularly infectious morbidity for mother and neonate, have also not been adequately assessed. UK guidance therefore recommends individualized decision making by a senior obstetrician, with particular caution in cases presenting ≥ 24 weeks.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


