CHAPTER 38 Premalignant disease of the genital tract
The Cervix
In 1886, Sir John Williams described eight cases of cervical cancer, one of which was equivalent to carcinoma in situ:
The terminology of cervical premalignancy
The premise that cervical squamous premalignancy was a continuum underpinned the concept of cervical intraepithelial neoplasia (CIN), which was suggested by Richart (1967). The usefulness of this system was limited by significant interobserver variability in the diagnosis and grading of CIN, particularly in differentiating CIN1 from human papilloma virus (HPV) lesions, and separating CIN1 from CIN2 lesions. Furthermore, there is no clear evidence that CIN3 arises from earlier lesions.
Pathology of cervical premalignancy
Cervical intraepithelial neoplasia
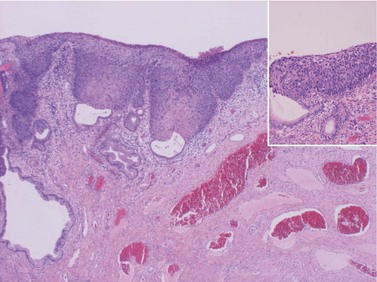
CIN may affect the gland crypts as well as the surface epithelium (Figure 38.1). It is recognized that the degree and depth of crypt involvement increases with the grade of CIN. Histological assessment of crypt involvement in women with CIN3 has shown a mean depth of 1–2 mm, with a maximum of 5.22 mm and a mean ±3 standard deviations (99.7%) of 3.8 mm (Anderson and Hartley 1980). These figures suggested that treatment of ectocervical lesions to a depth of 7 mm should be sufficient to eradicate most CIN.
Cervical glandular intraepithelial neoplasia
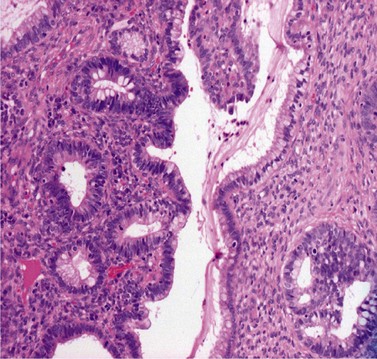
CGIN is characterized by columnar cells with hyperchromatic nuclei and stippled chromatin (Figure 38.2). The nuclei show increased stratification and abnormal mitotic figures with loss of normal mucin. In some cases, the whole of a gland may be involved, but the lesion often occurs as a sharply demarcated area. It may be multifocal. CGIN is often associated with goblet cells or intestinal metaplasia of the endocervical cells. In two-thirds of cases, there are associated squamous abnormalities, and CGIN is often serendipitously discovered in the management of these abnormalities.
Pathogenesis of cervical premalignancy
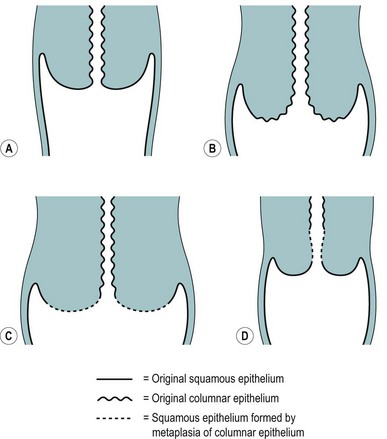
The position of the SCJ is influenced by the hormonal changes that occur during a woman’s life (Figure 38.3). With the onset of puberty, the uterus enlarges and the cervix swells with a resultant eversion, exposing columnar epithelium to the acid environment of the vagina. This induces metaplasia in the exposed columnar epithelium, resulting in the development of metaplastic squamous epithelium. This area of transformation is termed the ‘cervical transformation zone’ (cervical TZ), and it is within this area that preneoplastic changes can occur with the development of CIN. It is thought that these dysplastic changes occur at the time of metaplasia, indicating that this is the time when the cervix is most vulnerable to potential carcinogenic factors, such as HPV and other cofactors.
These considerations have important clinical significance with regards to detecting and treating CIN, as these changes occur within the cervical TZ and it is accessible. Furthermore, the recognition that the development of precancerous changes can occur early in a woman’s sexual and reproductive life provided some indication that these events were potentially associated with some form of environmental exposure related to sexual activity. In other words, most of the identified risk factors for CIN are thought to be largely surrogate markers of HPV infection (Table 38.1).
Table 38.1 Risk factors for cervical intraepithelial neoplasia
Cervical intraepithelial neoplasia
The malignant potential of CIN3 was shown by McIndoe et al (1984) in a crucial paper. This indicated that approximately 30% of women with CIN3 would develop invasive cancer over a 20-year period.
The authors’ rationale for treating CIN3 is based upon this paper, which implies that when CIN3 is discovered, it should be treated. However, there are caveats. Firstly, the patients in this study had large carcinoma in situ lesions, and can we be sure that the undoubted premalignant potential for the lesions managed in this paper are shared by patients with smaller lesions that have small foci of CIN3? For example, it has been estimated that perhaps one-third of cases with CIN3 regress (Östör 1993).
Aetiology of cervical premalignancy
The epidemiological risk factors for both squamous and glandular cervical premalignant lesions are similar and include young age at first intercourse and multiple sexual partners. It is now well established that infection with oncogenic high-risk HPV types is the central causal factor in the development of cervical neoplasia (Walboomers et al 1999).
HPVs are small double-stranded DNA viruses which have an icosahedral protein capsid (Figure 38.4). They are typed according to the DNA sequence homology in particular genes, specifically L1 (which codes for the viral capsid) and E6 and E7 (which have important carcinogenic functions). Nearly 30 HPV types can infect the genital tract and can be classified into high-, intermediate- and low-risk oncogenic types. HPV types 16 and 18 are by far the most common high-risk types, accounting for 60% of HPV-positive invasive cervical cancers.
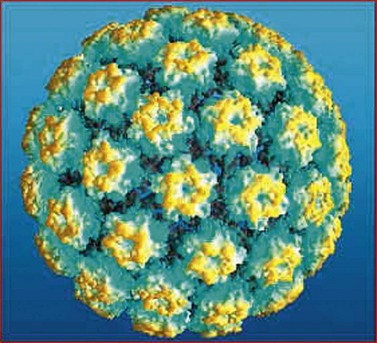
Figure 38.4 Model of human papilloma virus showing the arrangement of capsid proteins.
Copyright © 2009, Re-used with the permission of The Health and Social Care Information Centre. All rights reserved.
Prevention of cervical premalignancy
Primary prevention using immunization
Several phase III trials (Ault 2007) have shown that more than 90% of persistent HPV 16/18 infections can be prevented for up to 5 years after vaccination, and that more than 90% of precancerous lesions can be prevented in subjects who were HPV negative prior to vaccination. The long-term effects on cervical cancer incidence will require another 10–20 years of follow-up.
Detection of cervical premalignancy
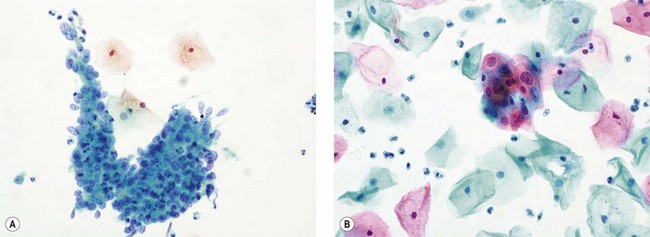
Cervical cytology
The recognition that cervical cytology could be used to detect precancerous change led to the introduction of cervical cytology as a screening test (Figure 38.5). Early detection and treatment can prevent the development of 75% of cancers. Whilst cytology is used to detect women at risk of having cervical premalignancy, most abnormalities are not precancerous. Only a small proportion of women with abnormal smears would develop cancer, although these women are high risk compared with the normal population. There is therefore huge potential for overtreatment unless one can accurately select which lesions require treatment.
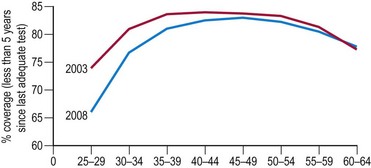
In the UK, the incidence of cervical cancers has halved since the National Health Service’s (NHS) cervical screening programme was introduced in 1988. The NHS cervical screening programme is highly organized. In the UK, women aged 25–65 years are invited for screening every 3 or 5 years. It is thought that screening under the age of 25 years may do more harm than good as cervical cancer is rare in this age group (Sasieni and Adams 1999). There are clear service guidelines, effective data collection systems using a number of mandatory returns from cytological laboratories, and internal and external quality assurance systems. Target population coverage is the key to success. The programme aims for coverage of over 80% of the target population, but there has been a worrying fall in levels in recent years, falling as low as 66% in women aged 25–30 years (Figure 38.6).
HPV detection assay
It is likely that the combination of prophylactic HPV vaccination and the use of HPV testing as a primary test will be the most cost-effective strategy. Assuming a protective effect of prophylactic HPV vaccination of 15–20 years, nationwide prepubertal vaccination may allow delaying the onset of the cervical screening programme to 30 years instead of 25 years as is the current guidance (Bulkmans et al 2007).
Colposcopy
Colposcopic examination
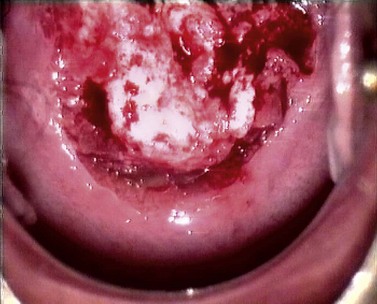
At the initial examination, obvious macroscopic abnormality is sought, including leukoplakia, viral condylomata and invasion. Invasion is associated with the surface of the cervix appearing raised or ulcerated (Figure 38.7). Atypical vessels seen on invasive lesions run a bizarre course and are often corkscrew- or comma-shaped (Figure 38.8). Condylomata are usually obvious from their regular frond-like surface (Figure 38.9).