Vulva
Studies on the natural history of VIN lesions are much fewer than for CIN. Partly because of the paucity of studies, the relationships between VIN and invasive squamous cell carcinoma of the vulva appear to be less straightforward than those documented between CIN and invasive squamous cell carcinoma of the cervix. Unlike the cervix, in which the majority of carcinomas are associated with a CIN lesion, only a third of invasive vulvar squamous carcinomas have a coexisting VIN 3 lesion. A review of six published follow-up studies found that only 16 of 330 patients (4.8%) with VIN progressed to invasive cancer. What is generally not emphasized when the data are discussed is that most of the patients in these follow-up studies were treated for their VIN or followed for relatively short periods of time.
RISK FACTORS FOR THE DEVELOPMENT OF LOWER GENITAL TRACT CANCERS AND PREINVASIVE LESIONS OF THE LOWER GENITAL TRACT
KEY POINTS
- Smoking and number of sexual partners are the greatest risk factors for cervical cancer and its precursors.
- HPV infection is acquired through sexual contact and is necessary for the development of cervical cancer and its precursors.
- The majority of HPV-infected women spontaneously clear their infections within 1 to 2 years.
Cervix
Risk Factors
A large number of epidemiologic studies have analyzed risk factors for the development of cervical cancer and its precursors. Although the risk factors are similar for both cervical cancer and its precursors, the association with the risk factors is generally much stronger for cervical cancer than for precursor lesions. The major risk factors found in most studies are markers of sexual behavior such as number of sexual partners, early age of first pregnancy and first intercourse, sexually transmitted diseases, and parity. In addition, lower socioeconomic class, cigarette smoking, oral contraceptive use, specific HLA-DR haplotypes, and immunosuppression from any cause are associated with both cervical cancer and its precursors.
Human Papillomavirus
Over the last 15 years, considerable evidence has been accumulating rapidly to implicate HPV in the pathogenesis of cervical cancer and its preinvasive precursors. Epidemiologic and molecular studies have found that there is a consistent and strong relationship between HPV infection and cervical neoplasia, that the temporal sequence between the infection and the development of cancer is correct, that the association between HPV and cervical cancer is relatively specific, and that the epidemiologic findings are consistent with the natural history and biologic behavior of HPV infections and cervical cancer.
Epidemiologic studies clearly demonstrate that there is a strong and consistent association between specific types of HPV DNA and invasive cervical cancer and its precursor lesions and that exposure to HPV precedes the development of cervical disease. When combined with the enormous body of molecular evidence demonstrating a role for HPV in the development of cervical cancer and CIN, these findings clearly indicate that HPV infection, acquired through sexual contact, is a “necessary cause” of both CIN and invasive cervical cancer. Based on these data, the International Agency for Research on Cancer of the World Health Organization has classified HPV 16 and 18 and all of the other “high-risk” types of HPV as carcinogens in humans.
Smoking and Oral Contraceptives
In addition to risk factors associated with sexual behavior, several other risk factors such as low socioeconomic class and cigarette smoking have also been associated with the development of cervical cancer. The use of combined oral contraceptives has also been found to be a risk factor for the development of cervical cancer and its precursors in some studies. A recent reanalysis of epidemiologic studies involving over 50,000 women confirmed an increase risk of cervical cancer in oral contraceptive users, though the confounding effects of sexual behavior may not have been fully considered.
Immunosuppression
Immunosuppression is another risk factor for the development of both CIN and cervical cancer. In studies of patients with renal transplants, transplant recipients have a relative risk of 13.6 for the development of cervical carcinoma in situ compared to women in the general population. Over the last decade, it has become widely accepted that there is also an association between cervical disease and infection with the human immunodeficiency virus (HIV). Among women in New York City, the standardized incidence ratio for cervical cancer is 9.2 times higher in HIV-infected women compared to noninfected women. It is clear that the invasive cervical cancers that do develop in HIV-infected women act aggressively and respond poorly to standard forms of therapy. Invasive cervical cancer was designated in 1993 as an AIDS case–defining illness by the Centers for Disease Control and Prevention.
HUMAN PAPILLOMAVIRUS
Classification
Papillomaviruses are a diverse group of viruses that are widely distributed in mammals and birds. Papillomavirus isolates are traditionally described as types. The characteristic features of papillomaviruses are a double-stranded, circular DNA genome of about 8,000 base pairs, a nonenveloped virion, and an icosahedral capsid. To date, 118 papillomavirus types have been completely described. Over 30 types of HPV that infect the anogenital tract have been described. These different types of HPV tend to be associated with different types of lesions. HPV 6 and 11 are the most common HPV types found in association with exophytic condylomas of the male and female anogenital tracts in adults.
Almost half of all CIN 2,3 lesions are associated with HPV 16. A meta-analysis of the distribution of HPV types in CIN 2,3 lesions recently concluded that HPV 16 is identified in 45.3%, HPV 18 in 6.9%, and HPV 31 in 8.6%. Based on their associations with benign epithelial proliferations, high-grade cancer precursors, or invasive cancers of the vulva, vagina, anus, and cervix, HPVs can been categorized into “high risk” or “low risk” based on the relative risk of being associated with cancer (Table 4.2).
Table 4.2 Classification of Anogenital HPV Types

aClassified as “low risk” based on other data.
Reprinted with permission from: Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518.
PREVALENCE AND TRANSMISSION OF HPV INFECTIONS
The prevalence of genital tract HPV infections and the frequency of exposure to HPV in the general population vary widely in different studies. The recent National Health and Nutrition Examination Study (NHANES) found that the overall prevalence of HPV infection was 42.5% in women aged 14 to 49 years. The prevalence is highest in college-age women (Fig. 4.1). It is clear that the prevalence of HPV infection in the general population is consistently higher than the prevalence of CIN, by about one order of magnitude. The majority of women who are infected with HPV will never develop CIN 2,3 or cancer.
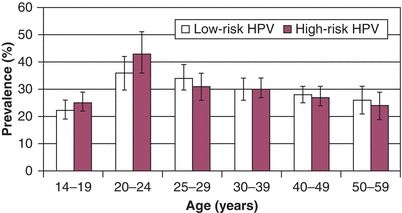
Figure 4.1 US prevalence of HPV infections in women. Source: Reprinted with permission from Harir S, Unger ER, Sternberg M, et al. Prevalence of genital human papillomavirus in the United States, the National Health and Nutrition Examination Survey, 2003–2006. J Infect Dis. 2011;204:566–573.
Both genital and nongenital HPV appear to be transmitted predominately through close “skin-to-skin” or “mucosa-to-mucosa” contact, and transmission is facilitated by minor trauma at the site of inoculation. The importance of sexual transmission is highlighted by studies of young women initiating sexual activity. In a study of 604 women attending a university, HPV DNA was detected by PCR in only 3% of women reporting no prior vaginal intercourse, 7% of women with 1 male sexual partner, 33% of women with 2 to 4 partners, and 53% of women with 5 or more male partners.
OUTCOME AFTER EXPOSURE TO HPV
Infection with a high-risk anogenital HPV in most women leads to a transient productive viral infection that lasts for a period of months to years. Approximately one third of these women develop low-grade cytologic or histologic changes. Nevertheless, the vast majority of HPV-infected women spontaneously clear their infections within 1 to 2 years. Clearance appears to be mediated by cellular immune responses. Prospective follow-up studies report that less than 20% of women remain DNA positive after 24 months of follow-up (Table 4.3).
Table 4.3 HPV Persistence
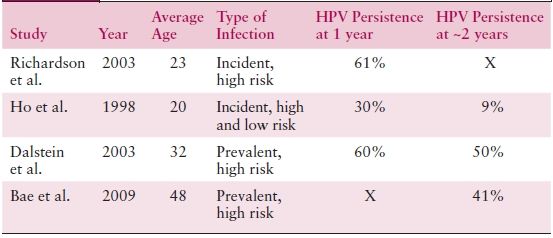
Sources: Richardson H, et al. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomarkers Prev. 2003;12:485–490; Ho GY, et al. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–428; Dalstein V, et al. Persistence and load of high-risk HPV are predictors for development of high-grade cervical lesions: A longitudinal French cohort study. Int J Cancer. 2003;106:396–403; Bae J, et al. Natural history of persistent high-risk human papillomavirus infections in Korean women. Gynecol Oncol. 2009;115:75–80.
It is currently unknown what proportion of women who appear to spontaneously clear their HPV infections and become HPV DNA negative truly clear the infection from the anogenital tract and what proportion continue to have a latent, undetectable HPV infection.
Reactivation of latent infections could explain the increase in the prevalence of HPV among cytologically negative older women (Fig. 4.2).
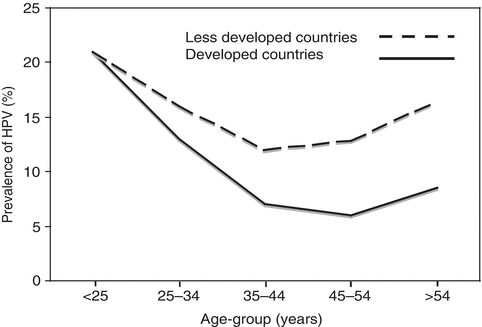
Figure 4.2 Estimate of prevalence of HPV DNA positivity in women with normal cervical cytology. The estimate is based on a meta-analysis that included published studies from all regions of the world. Source: Adapted with permission from de Sanjose S, Diaz M, Casellsague X, et al. Worldwide prevalence and genotype distribution of cervical HPV DNA in women with normal cytology: A metaanalysis. Lancet Infect Dis. 2007;7(7): 453–459.
Although spontaneous clearance can continue to occur even after 24 months, clinically significant persistent infections should probably be defined as infections that last at least 2 years. Long-term, stable persistent infections appear to occur in only about 10% of infected individuals. Although newer studies suggest that CIN 2,3 lesions can develop within a relatively short period of time after initial infection, lesions that do not persist for at least 2 years are unlikely to have clinical significance.
CYTOLOGIC SCREENING FOR CERVICAL CANCER AND ITS PRECURSORS
KEY POINTS
- Cervical cytology has reduced the incidence of cervical cancer by almost 80% in the United States.
- Cervical screening should begin at age 21 years regardless of age at first sexual intercourse.
- Liquid-based Pap smears have the advantage of providing reflex HPV testing in women with atypical squamous cells of undetermined significance (ASCUS).
Cytology-based cervical cancer screening programs were first introduced in the mid-20th century and are now widely recognized as being able to reduce both the incidence of, and mortality from, invasive cervical cancer. Strong evidence for their effectiveness comes from a comparison of incidence and mortality trends of invasive cervical cancer with screening activity in a given country or region.
Accuracy of Cervical Cytology
Despite the proven effectiveness of cervical cytology in reducing the incidence of cervical cancer, over the last several years, the accuracy of cervical cytology has been questioned. However, when the cytologic cutoff is low-grade squamous intraepithelial lesion (LSIL) and the end point is biopsy-confirmed CIN 2,3 or worse, the average sensitivity is 81% and the specificity is 77%.
A number of factors influence the false-negative rate of cervical cytology. Since the majority of CIN and cancers involve the transformation zone, sampling devices have been specifically designed to sample this area. Sampling the endocervical canal reduces the false-negative rate. This sample can be taken with an endocervical brush (e.g., cytobrush) or one of the newer collection devices such as a cell broom. Saline-moistened cotton-tipped applicators should not be used. Other important factors for reducing the false-negative rate are the rapid fixation of cells to prevent artifactual changes secondary to air-drying and the use of a cytology laboratory with stringent quality control standards.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


