CHAPTER 18 Polycystic ovary syndrome
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disturbance affecting women, and is a heterogeneous collection of signs and symptoms that, gathered together, form a spectrum of a disorder with a mild presentation in some women, and a severe disturbance of reproductive, endocrine and metabolic function in other women (Balen et al 1995). The pathophysiology of PCOS appears to be multifactorial. The definition of PCOS has been much debated. Key features include menstrual cycle disturbance, hyperandrogenism and obesity. There are many extraovarian aspects to the pathophysiology of PCOS, yet ovarian dysfunction is central. The international consensus definition of PCOS is the presence of two out of the following three criteria: (i) oligo-ovulation and/or anovulation; (ii) hyperandrogenism (clinical and/or biochemical); and (iii) polycystic ovaries, with the exclusion of other aetiologies of menstrual disturbance or hyperandrogenism (Fauser et al 2004). There is considerable heterogeneity of symptoms and signs amongst women with PCOS, and for an individual, these may change over time. Polycystic ovaries can exist without clinical signs of PCOS, expression of which may be precipitated by various factors, most predominantly an increase in body weight.
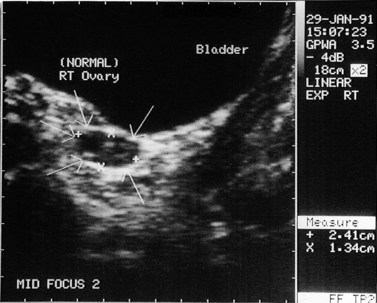
The morphology of the polycystic ovary is an ovary with 12 or more follicles measuring 2–9 mm in diameter and/or increased ovarian volume (>10 cm3) (Balen et al 2003) (Figures 18.1–18.5). Polycystic ovaries are commonly detected by pelvic ultrasound, with estimates of prevalence in the general population being in the order of 20–33% (Michelmore et al 1999). However, not all women with polycystic ovaries demonstrate the clinical and biochemical features which define PCOS. These include menstrual cycle disturbances, hirsutism, acne, alopecia and abnormalities of biochemical profiles, including elevated serum concentrations of luteinizing hormone (LH), testosterone and androstenedione. Obesity and hyperinsulinaemia are associated features, although only 40–50% of women with PCOS are overweight (Balen and Michelmore 2002). Presentation of PCOS is so varied that one, all or any combination of the above features may be present in association with an ultrasound picture of polycystic ovaries (Table 18.1).
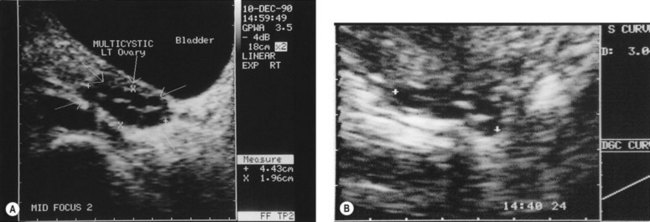
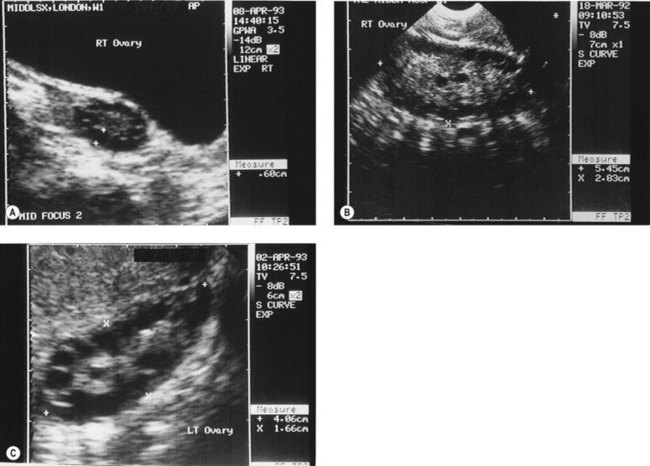
Figure 18.3 (A) Transabdominal scan of a multicystic ovary. (B) Transvaginal ultrasound scan of a multicystic ovary.
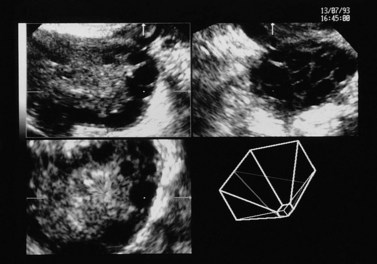
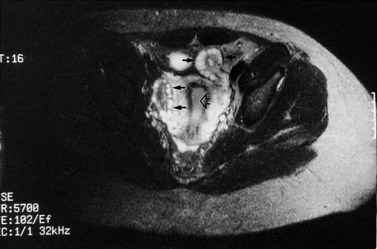
Figure 18.4 Three-dimensional transvaginal ultrasound scan of a polycystic ovary.
Courtesy of Dr A. Kyei-Mensah.
Table 18.1 The spectrum of clinical manifestations of polycystic ovary syndrome
| Symptoms | Serum endocrinology | Possible late sequelae |
|---|---|---|
| Obesity | ↑ Androgens (testosterone and androstenedione) | |
| Menstrual disturbance | ↑ Luteinizing hormone | Hypertension |
| Infertility | Cardiovascular disease | |
| Hyperandrogenism | ||
| Asymptomatic |
Pathogenesis
The pathogenesis of polycystic ovaries and PCOS is still being elucidated, but the heterogeneity of presentation of PCOS suggests that a single cause is unlikely. Some genetic studies have identified a link between PCOS and disordered insulin metabolism, and indicate that PCOS may be the presentation of a complex genetic trait disorder (Franks et al 2001). PCOS runs in families, with approximately half of first-degree relatives (i.e. sisters, mothers and daughters) also being affected; incidentally, male relatives also show an increased rate of metabolic problems. The features of obesity, hyperinsulinaemia and hyperandrogenaemia, which are commonly seen in PCOS, are also known to be factors which confer an increased risk of cardiovascular disease and type 2 diabetes (Rajkowha et al 2000). There are studies which indicate that women with PCOS have an increased risk for these diseases which pose long-term risks for health.
PCOS appears to have its origins during adolescence and is thought to be associated with increased weight gain during puberty. However, the polycystic ovary gene(s) has not yet been identified, and the effects of environmental influences such as weight changes and circulating hormone concentrations, and the age at which these occur, are still being unravelled. Prior to puberty, there appears to be two periods of increased ovarian growth. The first is at adrenarche in response to increased concentrations of circulating androgens, and the second is just before and during puberty due to rising gonadotrophin levels, and the actions of growth hormone, insulin-like growth factor-1 (IGF-1) and insulin on the ovary. Sampaolo et al (1994) reported a study of 49 obese girls at different stages of puberty, comparing their pelvic ultrasound features and endocrine profiles with 35 age- and pubertal-stage-matched controls. They found that obesity was associated with a significant increase in uterine and ovarian volume. They also found that obese postmenarcheal girls with polycystic ovaries had larger uterine and ovarian volumes than obese postmenarcheal girls with normal ovaries. Sampaolo et al concluded that obesity leads to hyperinsulinism, which causes both hyperandrogenaemia and raised IGF-1 levels, which augments the ovarian response to gonadotrophins. This implies that obesity may be important in the pathogenesis of polycystic ovaries, but further study is required to evaluate this. It is known that obesity is not a prerequisite for PCOS. After menarche, it is common for the menstrual cycle to be erratic for several months. If the irregularity persists beyond 2 years, there is a high chance that the adolescent girl has PCOS.
Investigations
Endocrine profile
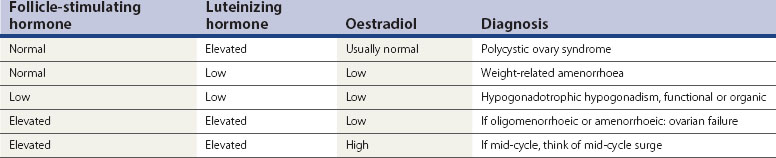
Women with PCOS usually have a normal serum FSH concentration (Table 18.2). LH is the second gonadotrophin which, like FSH, is released by the gonadotrophs in the anterior pituitary gland, under the influence of pulsatile release of gonadotrophin-releasing hormone (GnRH). The differential control of FSH and LH secretion relies upon the need for priming of the pituitary by oestradiol before it will become responsive to GnRH and release LH. FSH secretion, on the other hand, is under tonic inhibitory control by inhibin acting in a negative feedback loop from the ovaries. Therefore, in times of oestrogen deficiency, such as weight-related amenorrhoea, LH concentrations in the circulation are lower than FSH, whilst the mid-cycle surge that is primed by rising oestradiol secretion from the ovary results in a greater release of LH than FSH.
An elevated serum concentration of LH in the follicular phase of the cycle suggests that the patient has PCOS, usually associated with a concentration of more than 10 IU/l in the early to mid-follicular phase of the cycle. In a series of over 1700 women with PCOS, approximately 40% of patients were found to have an elevated serum concentration of LH, which was associated with a significantly higher risk of infertility than in those with normal LH levels (Balen et al 1995). Other causes of an elevated LH serum concentration are the mid-cycle surge and ovarian failure (Table 18.2).
Hyperinsulinaemia
The association between insulin resistance, compensatory hyperinsulinaemia and hyperandrogenism has provided insight into the pathogenesis of PCOS. The cellular and molecular mechanisms of insulin resistance in PCOS have been investigated extensively, and it is evident that the major defect is a decrease in insulin sensitivity secondary to a postbinding abnormality in insulin-receptor-mediated signal transduction, with a less substantial, but significant, decrease in insulin responsiveness (Dunaif 1997). It appears that decreased insulin sensitivity in PCOS is potentially an intrinsic defect in genetically susceptible women, since it is independent of obesity, metabolic abnormalities, body fat topography and sex hormone levels.
Although the insulin resistance may occur irrespective of body mass index (BMI), the common association between PCOS and obesity has a synergistic deleterious impact on glucose homeostasis, and can worsen both hyperandrogenism and anovulation. An assessment of BMI alone is not thought to provide a reliable prediction of cardiovascular risk. It has been reported that the association between BMI and coronary heart disease almost disappeared after correction for dyslipidaemia, hyperglycaemia and hypertension. Some women have profound metabolic abnormalities in the presence of a normal BMI, and others have few risk factors with an elevated BMI. It has been suggested that rather than BMI itself, it is the distribution of fat that is important, with android obesity being more of a risk factor than gynaecoid obesity. Hence the value of measuring the waist:hip ratio or waist circumference, which detect abdominal visceral fat rather than subcutaneous fat. It is the visceral fat which is metabolically active and, when increased, results in increased rates of insulin resistance, type 2 diabetes, dyslipidaemia, hypertension and left ventricular enlargement. There is a closer link between waist circumference and visceral fat mass, as assessed by computer tomography, than waist:hip ratio or BMI (Lord and Wilkin 2002). Waist circumference should ideally be less than 79 cm, whilst a measurement of greater than 87 cm carries a significant risk. Exercise has a significant effect on reducing visceral fat and reducing cardiovascular risk; indeed, a 10% reduction in body weight may equate to a 30% reduction in visceral fat.
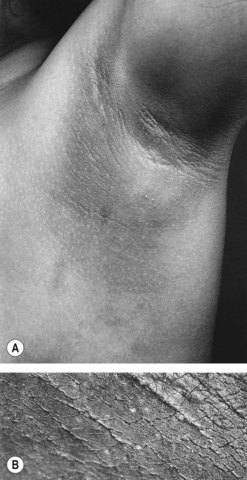
Insulin acts through multiple sites to increase endogenous androgen levels. Increased peripheral insulin resistance results in a higher serum insulin concentration. Excess insulin binds to IGF-1 receptors which enhances androgen production by theca cells in response to LH stimulation. Hyperinsulinaemia also decreases the synthesis of SHBG by the liver. Therefore, there is an increase in the serum free testosterone concentration, and consequent peripheral androgen action. Intraovarian androgen excess is responsible for anovulation by acting directly on the ovary, promoting the process of follicular atresia. This latter process is characterized by apoptosis of granulosa cells. As a consequence, there is an increasingly larger stromal compartment, which retains LH responsiveness and continues to secrete androgens. Hyperinsulinaemia also stimulates trophic changes in the skin that results in acanthosis nigricans in the skin creases (Figure 18.6).
Insulin resistance can be measured by a number of expensive and complex tests, but it is not necessary to measure it routinely in clinical practice; it is more important to check for impaired glucose tolerance. Simple screening tests for risk of impaired glucose tolerance (IGT) include an assessment of BMI and waist circumference. If the fasting blood glucose is less than 5.2 mmmol/l, the risk of impaired glucose tolerance is low. The 2-h standard 75 g oral glucose tolerance test may be conducted in those at high risk (BMI >30 kg/m2 in Caucasian women and >25 kg/m2 in women from South Asia, who have a greater degree of insulin resistance at a lower body weight) (Table 18.3).
Heterogeneity of PCOS
A few years ago, the author reported a large series of women with polycystic ovaries detected by ultrasound scan (Balen et al 1995). All of the 1871 patients had at least one symptom of PCOS. Thirty-eight percent of the women were overweight (BMI >25 kg/m2). Obesity was significantly associated with an increased risk of hirsutism, menstrual cycle disturbance and an elevated serum testosterone concentration. Obesity was also associated with an increased rate of infertility. Twenty-six percent of patients with primary infertility and 14% of patients with secondary infertility had a BMI of more than 30 kg/m2. Approximately 30% of the patients had a regular menstrual cycle, 50% had oligomenorrhoea and 20% had amenorrhoea. In this study, the classical endocrine features of raised serum LH and testosterone were found in 40% and 30% of patients, respectively. Ovarian volume was significantly correlated with serum LH and testosterone concentrations. Other studies have reported correlation between markers of insulin resistance and ovarian volume and ovarian stromal echogenicity, which in turn have been correlated with androgen production.
National and racial differences in expression
Approximately 75–80% of women with polycystic ovaries have signs or symptoms of PCOS. Thus, in the UK, where it has been reported that up to 33% of women have polycystic ovaries (Michelmore et al 1999), 20–25% of women may have a degree of PCOS, albeit mild in many cases. There are large national and ethnic variations in the expression of PCOS, with women from the Far East having little in the way of hirsutism, whilst those with dark hair from Mediterranean and Middle Eastern or South Asian countries have a greater degree of expression. The highest reported prevalence of polycystic ovaries has been 52% among South Asian immigrants in Britain, of whom 49% had menstrual irregularity (Rodin et al 1998). It was also shown that South Asian women with polycystic ovaries had a comparable degree of insulin resistance to controls with established type 2 diabetes. Generally, there has been a paucity of data of the prevalence of PCOS among women of South Asian origin, both among migrant and native groups. Type 2 diabetes and insulin resistance have a high prevalence among indigenous populations in South Asia, with a rising prevalence among women. Insulin resistance and hyperinsulinaemia are common antecedents of type 2 diabetes, with a high prevalence in South Asians. Type 2 diabetes also has a familial basis, inherited as a complex genetic trait that interacts with environmental factors, chiefly nutrition, commencing from fetal life. It has been shown that ethnic variations in the overt features of PCOS (symptoms of hyperandrogenism, menstrual irregularity and obesity) in women of South Asian descent are linked to the higher prevalence and degree of insulin resistance in South Asians. It has also been shown that South Asians with anovulatory PCOS have greater insulin resistance and more severe symptoms of PCOS than anovular White Caucasians with PCOS (Wijeyaratne et al 2002).
The question remains as to whether differences in expression of PCOS are due to dietary and lifestyle factors or to genetic variations in hormone actions, such as polymorphisms in gonadotrophin subunits or receptor function (affecting the expression of androgens, gonadotrophins or insulin). A full discussion of the genetics of PCOS is beyond the scope of this chapter, and there are a number of candidate genes that have been proposed (see Franks et al 2001). It may be that some families or racial groups have genetic differences that affect the expression or presentation of PCOS.