40 Pleural Effusions and Pneumothorax
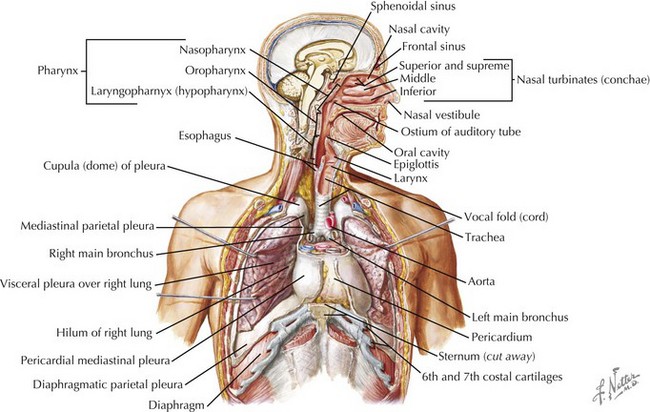
Pleural effusions and pneumothoraces occur as a result of structural and mechanical abnormalities of the pleural space. Abnormalities of the pleural space are an important cause of morbidity and mortality in infants and children worldwide, and the number of children who develop clinically significant pleural effusions is increasing. Pleural effusions are the result of excessive fluid accumulation in the pleural space, and pneumothoraces occur as a result of the accumulation of air within the pleural space. To better understand the pathophysiology of pleural effusions and pneumothoraces, it is essential to understand the anatomy of the pleural space. The pleural space is a potential anatomic space, approximately 10 to 20 µm wide, located between the visceral and parietal pleurae. The visceral pleura lines the surface of the lung parenchyma, including the interlobar fissures, and the parietal pleura lines the inner surface of the chest wall, mediastinum, and diaphragm (Figure 40-1). The pleural space contains a small amount of fluid (0.3 mL/kg body weight) that is in equilibrium between the amount of fluid formed (filtered) and the amount removed (absorbed).
Pleural Effusions
Evaluation and Management
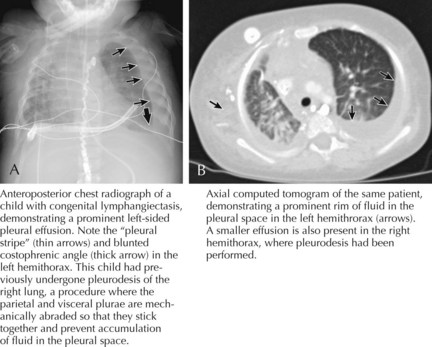
Children with the clinical history and findings suggestive of a pleural effusion should be evaluated with an upright chest radiograph and a lateral decubitus view (Figure 40-2). Performing radiographic examinations with the child in multiple positions helps to demonstrate a shift in the effusion with position changes. These radiographic images can help in making the diagnosis of pleural effusion and in determining the need for thoracocentesis or chest tube placement.
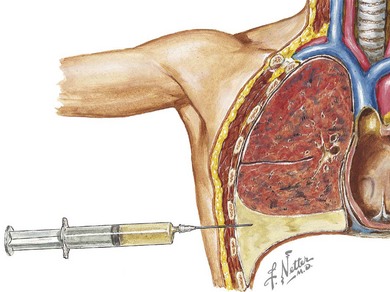
When infection is in the differential diagnosis, pleural fluid aspiration via thoracocentesis should be performed if at least 1 cm of fluid is seen on decubitus radiographs to determine the type of effusion (i.e., infectious exudate, empyema, hydrothorax, hemothorax, or chylothorax) before starting therapy (Figure 40-3). Certain laboratory studies should always be performed on the pleural fluid aspirate. Additional laboratory studies should also be obtained during the evaluation of a child with a parapneumonic effusion or empyema (Table 40-1). Microbiologic studies of the pleural fluid can identify a causative organism, and the analysis of pleural fluid is helpful in guiding therapeutic options. Pleural fluid can be obtained through thoracocentesis, aspiration under ultrasonographic guidance, or through video-assisted thoracoscopic surgery (VATS), depending on the presence of loculations or the availability of pediatric surgeons trained in VATS.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree