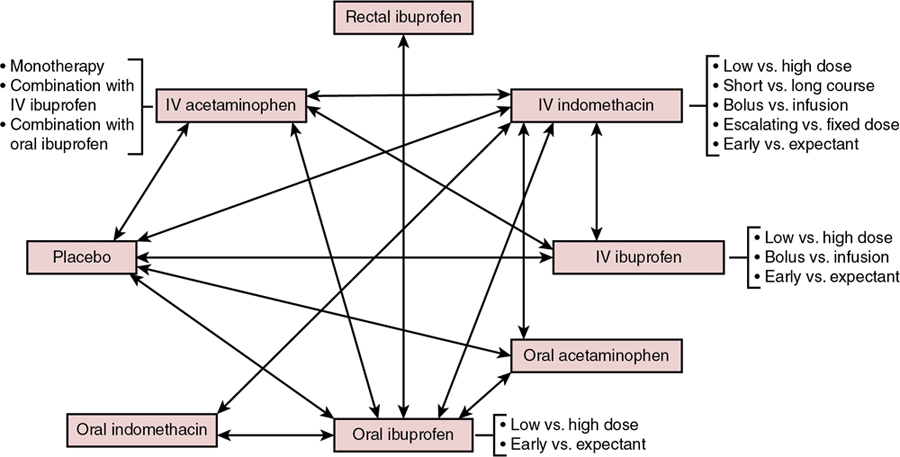
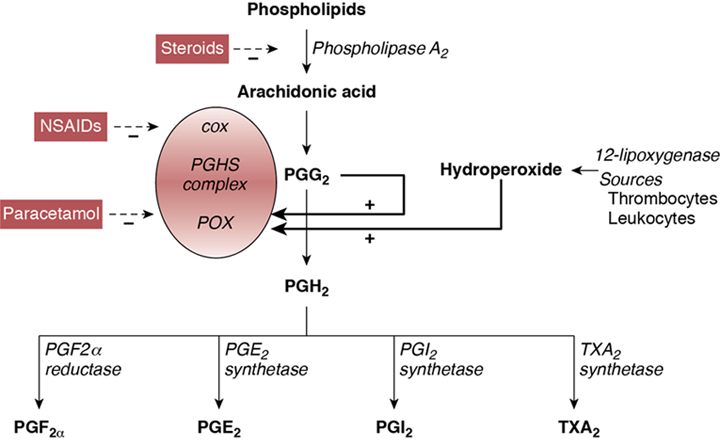
Souvik Mitra, Prakesh S. Shah Key points This chapter reviews the epidemiology and current state of pharmacological management (prophylactic and therapeutic) of the patent ductus arteriosus (PDA) in the very preterm neonate. Until now, the optimal management of PDA has been controversial in the scientific community, with no clear consensus or generally accepted guidelines for management. Most of the dispute stems from several sources: the natural history of PDA, the hemodynamic significance of PDA, especially concerning its impact on end-organ perfusion and its potential long-term consequences on neurodevelopment, variable efficacy of the available treatments, unpredictable side effects of pharmacological and surgical therapy, and the lack of information on patient-important clinical outcomes and long-term neurodevelopmental outcomes associated with treatment. The wide variations in PDA management reported worldwide reflect our poor understanding of these uncertainties. While efforts are underway to expand our current understanding of the condition, clinicians should continue to weigh the risks and benefits of different treatment options when deciding the correct clinical course of action. Apart from pharmacological therapy, conservative management and mechanical closure (surgical and percutaneous transcatheter route) of PDA are employed in the management of PDA. Conservative management approaches may range from watchful waiting to non-pharmacological shunt modulation strategies such as increasing the positive end-expiratory pressure. In contrast, pharmaceutical agents for PDA therapy are specifically used to stimulate ductal closure. Pharmacological agents stimulate PDA closure via inhibition of prostaglandin production, which play a significant role in maintaining ductal patency in utero and during the first 1–2 postnatal weeks. These agents are specifically designed to target either cyclooxygenase (COX) or peroxidase (POX), the second and third enzymes in the process of prostaglandin synthesis, respectively. These agents include the COX inhibitors indomethacin and ibuprofen and the POX inhibitor acetaminophen (paracetamol) (Figure 17.1).1 Adding to the complexity of pharmacological management is the question of when to treat, which may include prophylactic (treat all without assessing), early asymptomatic (detect and treat early before PDA becomes significant during first week after birth), and symptomatic (treat when PDA becomes clinically and hemodynamically significant) treatment. Variations in the management of PDA in very preterm and very-low-birth-weight infants have been well reported in North America, Europe, Australia, and Asia. These differences have been described for all aspects of treatment, including if, when, and how to treat PDA. Surveys conducted over the past 20 years regarding the practitioner’s approach to treatment of PDA have yielded consistently variable results. In North America a survey of 100 Canadian neonatologists in 1998 revealed a wide variation in practices regarding management of PDA both within and between centers.2 Fluid restriction and indomethacin were used for treatment by 89% of neonatologists surveyed, while surgery was reserved for patients unresponsive to pharmacological agents or had contraindications. Use of echocardiography for diagnosis of PDA also varied among clinicians. Almost a decade later, 56 fellowship program directors in the United States were surveyed regarding the management of PDA.3 A quarter of respondents were using prophylactic indomethacin for prevention of interventricular hemorrhage (IVH) and 9% used indomethacin to treat asymptomatic PDA. In cases of persistent PDA three-quarters of respondents indicated use of more than one course of indomethacin, with nearly half reporting usage of two courses and half reporting three courses, if needed. Most respondents were keen on administering indomethacin below 2 weeks of age and used echocardiography criteria to determine PDA treatment. Hoellering and Cooke also surveyed neonatologists from Australia and New Zealand in 2007 for management of PDA in neonates of 28 weeks’ gestation or less or birth weights less than 1000 g.4 Expectant (or conservative) management of PDA was favored by 35% of clinicians, while 32% used echocardiographic-targeted prophylaxis, 16% used pre-symptomatic treatment, and 17% used a prophylactic approach; however, nearly half of participating units reported using more than one approach, often depending upon the preference of the individual practitioner. Interestingly, 86% of physicians used long courses of indomethacin and nearly one-quarter of respondents indicated that their approach was not influenced by published literature. This raises important questions regarding the level of effect that individual units or practitioners have on the outcomes of neonates with PDA. In a survey of 24 European Societies of Neonatology and Perinatology, Guimaraes and colleagues reported data on 45 responses from 19 countries.5 Most neonatal units used intravenous indomethacin (71%), followed by intravenous ibuprofen (36%) and oral ibuprofen (29%); some units reported use of multiple agents. Approximately half of the centers used a second course and one-quarter of them used a third course of pharmacotherapy in the event of persistent ductus. Nearly all (96%) units treated hemodynamically significant PDA (hsPDA), but a quarter also treated non-hsPDA. Only one neonatal unit preferred surgical ligation as the first-line therapy. In France nearly three-quarters of the 49 neonatal units surveyed between 2007 and 2008 reported the use of both clinical and echocardiography criteria to decide on treatment for PDA, whereas the remaining relied on echocardiography criteria alone.6 Most units also used echocardiography to diagnose PDA, but the criteria used to describe hsPDA differed. All units used ibuprofen to treat PDA, with most units using a standard course (see Section 3.2 on Ibuprofen below). Between one-half and two-thirds of centers indicated a tendency to use a second course when either the first course failed or if the duct reopened after successful closure. In the event of contraindications to medical treatment or ductal malformation, 39% of units considered surgery as the primary treatment. Irmesi et al. recently collated information from published randomized trials of PDA management around the world.7 They identified that treatment with indomethacin and ibuprofen was more prevalent in the United States and Canada, whereas ibuprofen was the most common agent used in Europe. Worldwide variations were further exposed in a recent international survey of investigators from 335 neonatal units in 11 high-income countries.8 The results indicated that Japan, Sweden, Finland, and the Tuscany region of Italy routinely perform echocardiogram screening for PDA. Treatment rates of pre-symptomatic PDA based on routine echocardiography results alone, regardless of a patient’s clinical status, was in the range of 6–85% (6% of units in Canada; 7% in Illinois;19% in Israel; 50% in Sweden; 40% in Spain; 27% in Switzerland; 50% in Australia and New Zealand; 40% in Finland; 75% in Tuscany, Italy; and 85% in Japan) among those who conduct echocardiography screening.8 Apart from the survey data described above, reports of actual practices in the management of PDA have recently been published. A recent cohort study from the iNEO (International Network for Evaluating Outcomes of Neonates) collaboration that included 39,096 infants born between 24 and 28 weeks’ gestation from across 6 countries (139 NICUs) demonstrated a wide variation in PDA treatment practices. While the overall PDA treatment rate was 45% in this cohort (13–77% by NICU), the observed to expected PDA treatment ratio ranged from 0.30 to 2.14.9 It was further noted that the relationship between the observed to expected PDA treatment ratio and primary composite outcome of death and severe neurological injury followed a U-shaped curve, suggesting that both low and high PDA treatment rates were associated with worse clinical outcomes.9 In another study Hagadorn and colleagues examined trends in the management of PDA in 19 US children’s hospitals between 2005 and 2014 and linked the data to neonatal outcomes.10 Approximately three-quarters of infants with PDA were treated with pharmacological management or surgery, with wide variation noted among hospitals. There was a steady decline in the number of neonates treated over the years, with the odds of treatment decreasing by 11% in each year of the study period. The trend of reducing treatment was temporally associated with a decline in mortality; however, bronchopulmonary dysplasia (BPD), periventricular leukomalacia, retinopathy of prematurity (ROP), and acute renal failure increased. In a population-based cohort study Edstedt Bonamy et al. evaluated regional variations and their relationship with outcomes in PDA management across 19 regions in 11 European countries between 2011 and 2012.11 The proportion of neonates ≤31 weeks’ gestation who received PDA treatment varied from 10% to 39% between units, and it was independent of perinatal characteristics of patients. Variations in PDA treatment rate were not associated with neonatal outcomes. In Canada conservative management of PDA in neonates between 23 and 32 weeks’ gestation increased from 14 to 38% between 2006 and 2012, while using pharmacotherapy alone and surgical treatment alone decreased from 58 to 49% and 7.1 to 2.5%, respectively, and both pharmacotherapy and surgical ligation dropped from 21 to 10% (all P < 0.01) during the same time period.12 With an increase in conservative management, there was a reduction in the composite outcome of mortality or major morbidity between 2009 and 2012 compared to 2006 and 2008; however, there remains the possibility of confounding by indication. Slaughter et al. attempted to adjust for residual confounding parameters by incorporating clinician preference-based variation in practice as an instrument in their analyses.13 They reported that though an infant’s chance of receiving pharmacotherapy increased by 0.84% for each 1% increase in the hospital’s annual pharmacotherapy rate for treatment of PDA, there was no association between pharmacotherapy and mortality and mortality or BPD in neonates of ≤28 weeks’ gestation. This finding suggests that conservative management of PDA may be a rational approach for a subset of preterm infants with PDA. However, more work needs to be done to identify the right patient population who would benefit from treatment of the PDA (Chapter 19). In a prospective cohort from France Rozé et al. evaluated the role of early screening (before day 3 of postnatal life) in neonates <29 weeks’ gestation.14 The authors determined that screened infants were more likely to be treated for PDA than unexposed infants (55% vs. 43%; odds ratio [OR] 1.62, 95% confidence interval [CI] 1.32–2.00). Screened neonates were at lower odds of mortality (OR 0.73, 95% CI 0.54–0.98) and pulmonary hemorrhage (OR 0.60, 95% CI 0.38–0.95). However, when instrumental variable analyses using unit preference for early screening were conducted, there was no statistically significant association between early screening and mortality (OR 0.62, 95% CI 0.37–1.04), suggesting that questions about screening, prophylaxis, and treatment could only best be answered in well-designed randomized trials. The variations reported above in both survey designs and in studies comparing the evolution of approaches and their relationship with outcomes indicate that the management of PDA is widely variable within neonatal units and at regional, national, and international levels. Pharmacological interventions for PDA can be divided into two groups: agents used for symptomatic treatment (i.e. management of pulmonary edema and heart failure) and agents that induce PDA closure. Based on symptoms associated with pulmonary hyperperfusion and heart failure, diuretics such as furosemide have been used to reduce overall fluid overload. Use of furosemide in the context of PDA management remains controversial. Animal studies suggest furosemide may stimulate renal production of prostaglandin E2, thereby contributing to ductal patency.15 Further, three randomized controlled trials (RCTs) exploring the role of furosemide in indomethacin-treated infants for symptomatic PDA have failed to demonstrate any benefit.16 In addition, loop diuretics, such as furosemide, are associated with various side effects, including electrolyte imbalance, nephrocalcinosis, and hearing impairment.17 However, a recent large observational study of 43,576 infants demonstrated that exposure to furosemide for any indication was associated with reduced odds of PDA treatment (adjusted odds ratio = 0.72, 95% CI 0.65–0.79).18 Therefore controversy remains on the role of furosemide in the management of symptomatic PDA. While the Canadian Pediatric Society suggests considering the use of furosemide for conservative management of symptomatic PDA, further research is required to strongly recommend its use.19 As previously mentioned, the three main pharmacological agents used to induce PDA closure are indomethacin, ibuprofen, and acetaminophen (paracetamol), each of which is described below. More than 80 RCTs have been conducted over the past 40 years exploring different dosages, duration, and routes of administration of these drugs. The complexity of management of PDA is summarized in Figure 17.2, where strategies that have been tested in randomized trials are delineated.20 Indomethacin is a potent and non-selective inhibitor of the COX enzyme and promotes PDA closure by inhibiting the synthesis of prostaglandins, including prostaglandin E2.21 The half-life of indomethacin is 4–5 hours longer on average in preterm neonates <32 weeks’ gestation compared to those >32 weeks’ gestation (17.2 ± 0.8 vs. 12.5 ± 0.5 hours) and thus prolonged accumulation can occur in very preterm neonates.22 Intravenous indomethacin has been used in various dosing regimens.21 Initial animal studies demonstrated that intravenous indomethacin in doses of 0.2–0.4 mg/kg substantially reduced PDA diameter in fetal lambs.23 Most RCTs conducted in preterm neonates have used three doses of 0.1–0.2 mg/kg/dose every 12–24 hours apart; however, many modifications of this strategy have been carried out. In one study dose escalation of indomethacin starting from 0.2 mg/kg and increasing to 1 mg/kg in non-responders resulted in a 98.5% PDA closure rate.24 It should be noted that higher doses are typically associated with increased risk of side effects. In another study a high-dose (0.2–0.5 mg/kg/dose) and low-dose (0.1 mg/kg/dose) regimen of indomethacin were compared in cases of persistent PDA following conventional treatment with the three conventional doses.25 Although the authors reported no difference in PDA closure rates (55% vs. 48%, respectively), the infants exposed to the higher dosage displayed increased rates of renal compromise and moderate to severe retinopathy.25 Though the most common route of administration of indomethacin is intravenous, it has been used orally, rectally, and intra-arterially. Six studies of oral use (ranging between 9 and 74 neonates) have reported PDA closure rates of 66–67%. Intra-arterial use in 26 neonates was successful in 76% of cases, whereas a 66% closure rate was observed in a small group of neonates treated either orally (n = 1) or rectally (n = 5).21 Both of these routes have not been widely used due to concerns of damage to mucosal layers from local direct effects of indomethacin, as well as the effects on prostaglandin synthesis inhibition affecting mucosal integrity of the gastrointestinal tract, especially the ileum. The usual duration for one course of indomethacin treatment is 48–72 hours. For some neonates, ductal closure can be a lengthy remodeling process and may need prolonged treatment. Five randomized trials compared PDA closure rates in neonates treated with a prolonged course of indomethacin versus routine treatment using a three-dose course and reported no difference in PDA closure rates but identified that an increased risk of necrotizing enterocolitis (NEC) was associated with longer indomethacin exposure (relative risk [RR] 1.87, 95% CI 1.07–3.27).26 Some practitioners advocate for an echocardiogram to be performed after the last dose (third dose of a routine course) and to continue treatment until the duct closes. However, based on concerns of adverse effects, a prolonged course is not recommended by most. Typically, indomethacin is administered as a slow infusion to avoid rapidly rising concentrations characteristic of bolus infusions. The potential impact of indomethacin concentration on cerebral, renal, and splanchnic blood flow has led to recommendations for infusion to be administered over a 20- to 30-minute period. Studies have reported reduced blood flow,27 and similar28 or higher closure rates (81% vs. 43%; P = 0.03),29 with bolus infusion compared with continuous infusion. However, as suggested by a previous systematic review, the evidence may be too limited to draw a conclusion regarding the superiority of either approach.30 Pharmacokinetic data from a small series of neonates suggest that in neonates who had lower plasma levels, faster clearance, and shorter half-life, the drug was less effective. In addition, there was a 20-fold variation in the plasma levels 24 hours after indomethacin administration among neonates.31 Indomethacin is a potent medication for PDA closure, with historically proven rates of ductal closure. Closure rates following an initial course vary from 48 to 98.5% depending on dose, duration, and method of administration.21,32,33 However, it is important to keep in mind that the majority of the placebo-controlled RCTs on indomethacin efficacy were conducted in the 1980s and 1990s and included more mature preterm infants with high spontaneous PDA closure rates. Therefore these numbers stated above may not be reflective of the PDA closure efficacy of indomethacin in extremely-low-gestational-age infants. Many times a repeat course is provided when either a PDA fails to close following the first course or reopens after initial closure. The reported success rates with a second course are approximately 40–50%.32 Very rarely is a third course of indomethacin attempted, as exposure to more than two courses has been associated with periventricular leukomalacia.34 It is unclear what pathological mechanisms play a role in this association but the indomethacin-induced prolonged decrease in cerebral blood flow might contribute to this phenomenon. The efficacy of indomethacin declines with decreasing gestational age and increasing postnatal age.35 Data regarding efficacy of indomethacin beyond 2 months of age suggest its ineffectiveness at this age.21 Similarly, it is unclear whether indomethacin is as useful in the treatment of periviable neonates of 22 and 23 weeks’ gestation as the efficacy decreases with decreasing gestational age. Indomethacin is used for prophylactic, early asymptomatic, and symptomatic treatment. Prophylactic use is employed in the first 24 hours after birth, irrespective of the presence of a PDA. Since most (85%) PDAs in VLBW infants have been shown to close spontaneously before hospital discharge, this strategy predisposes many neonates to overtreatment.36 The underlying basis of prophylactic administration is to reduce the incidence and/or severity of peri/intraventricular hemorrhage (P/IVH) through modulation of the PDA shunt effect in addition to lowering cerebral perfusion via mechanisms independent of its effect on prostaglandin synthesis. A systematic review and meta-analysis of 19 studies identified a significant reduction in the incidence of symptomatic PDA (RR 0.44; 95% CI 0.38–0.50) and need for surgical ligation of a PDA (RR 0.51; 95% CI 0.37–0.71) with prophylactic use compared to placebo.37 Indomethacin use was also associated with reduced rates of any P/IVH (RR 0.88; 95% CI 0.80–0.98) and severe P/IVH (RR 0.66; 95% CI 0.53–0.82). However, there was no improvement in neurodevelopmental outcomes during early childhood despite a reduction in the severity of P/IVH.37,38 This has created diverse opinions and practices regarding the use of prophylactic indomethacin in routine clinical settings. Certain subgroups, such as male sex, lack of antenatal corticosteroids, and extremely-low-gestational-age neonates, especially those born <27 weeks’ gestation, have been identified as potential candidates for prophylactic indomethacin. In addition, units with a higher underlying rate of P/IVH might use this approach as, in this situation; the benefits may outweigh the risks of prophylactic indomethacin administration. Using indomethacin during the “early asymptomatic phase” significantly lowers the number of patients exposed to the drug compared to prophylactic measures described above, yet several patients who would have had a spontaneous closure will still be exposed. A systematic review of three RCTs reported a reduction in symptomatic PDA (RR 0.36, 95% CI 0.19–0.68) and duration of oxygen therapy following indomethacin use in the early asymptomatic phase; however, there was no difference in any other neonatal complications and no assessment of long-term neurodevelopmental outcomes.39 A recent RCT compared treatment with indomethacin and a placebo within the first 12 hours after delivery in infants who positively screened for a “large” PDA, irrespective of their effects on hemodynamic status.40 The trial was stopped prematurely due to unavailability of indomethacin. There was a significant reduction in pulmonary hemorrhage (2% vs. 21%), early P/IVH (4.5% vs. 12.5%), and need for later medical treatment of PDA (20% vs. 40%) with early asymptomatic treatment. However, there was no difference in the primary outcome of death or abnormal head ultrasound findings.40 Another approach is to treat PDA when it becomes symptomatic or hemodynamically significant. This method prevents unnecessary exposure to indomethacin as far as the PDA is concerned. Early treatment is used to describe the administration of the medication within the first 5–7 postnatal days, while late treatment is considered to occur in the second week after birth. A meta-analysis of four trials conducted between 1980 and 1990 revealed a significant reduction in BPD (OR 0.39; 95% CI 0.21–0.76) and duration of mechanical ventilation in infants receiving early versus late symptomatic treatment.41 Furthermore, higher PDA closure rates by day 6 (73% vs. 44%; P < 0.001) and day 9 (91% vs. 78%; P <0.05) in early versus late treatment groups were shown in a randomized controlled trial.42 Infants treated early were more susceptible to side effects, such as lower urine output and higher creatinine levels, and experienced more severe adverse events. A recent systematic review of early treatment versus expectant management of the hemodynamically significant PDA showed that very early (defined as treatment initiated <3 days of age) or early treatment (defined as treatment initiated <7 days) with indomethacin was not associated with improvement in any clinically meaningful outcomes such as death, BPD, NEC, or need for PDA ligation, but it was associated with increased exposure to NSAIDs.43 Of note, a before-after observational study also showed that delayed initiation of treatment is feasible and may reduce exposure to pharmacologic agents; however, this approach may result in an increase in the combined outcome of death or BPD.44 Indomethacin produces alterations in cerebral, renal, and splanchnic blood flow in a concentration-dependent manner and thus can lead to side effects including cerebral ischemia, renal dysfunction, and gut ischemia, and it also impairs platelet aggregation. Reduction of blood flow in the renal arteries occurs within the first 30 minutes of indomethacin administration and continues for 2 hours.45 This can lead to elevations in urea and creatinine levels and even renal failure. Mucosal injury associated with indomethacin is secondary to effects on prostaglandin synthesis. Prophylactic indomethacin has been associated with increased odds of spontaneous intestinal perforation (SIP) independent of early feeding, in a Canadian cohort of extremely preterm infants (n = 4268; adjusted OR [aOR] 2.43, 95% CI 1.41–4.19).46 A recent individual patient data meta-analysis of RCTs further suggests that prophylactic indomethacin increases the risk of SIP when given concomitantly with prophylactic hydrocortisone.47 The risk of SIP may further be increased in extremely preterm infants who received antenatal corticosteroids within 7 days before birth (aOR 1.67, 95% CI 1.15–2.43).48 The association of indomethacin with NEC, on the contrary, is a subject of debate, since the occurrence of NEC could be due to the disturbance of blood flow in the presence of a hemodynamically significant PDA rather than indomethacin alone. Still, indomethacin is also known to reduce splanchnic blood flow during its administration, and therefore this cause-and-effect relationship remains unclear.27 Because of concerns regarding intestinal blood flow, feeding is either discontinued, held, or sustained depending upon the attending medical team’s preference; however, similar outcomes have been reported with each approach.49 Overall, there is high certainty of evidence from RCTs to demonstrate that indomethacin is effective in closing a symptomatic PDA compared to no treatment in preterm infants.50 Rate of successful PDA closure is approximately 70% with the first course and 50% with a repeat course. However, evidence is insufficient regarding the effects of indomethacin on other clinically relevant outcomes and medication-related adverse effects.50 Recommended use includes a routine course of three doses after excluding contraindications, followed by repeat use for persistent PDA, if clinically indicated. Varying side effects, concerns over the impact of oral use on immature gastric mucosa, and the availability of potentially safer alternatives have led to a decrease in indomethacin use for treatment of PDA. Finally, the decrease in the rate of P/IVH with the use of prophylactic indomethacin might serve as an indication of its use in a selected group of patients with higher risk of P/IVH (see Chapter 6). Ibuprofen is a non-selective COX inhibitor that does not alter cerebral perfusion and has a reduced effect on renal and gut perfusion. Ibuprofen inhibits COX-1 and COX-2 enzymes in a rapid and reversible manner. It is metabolized in the liver and excreted in urine, and thus physiological impairment of hepatic or renal function may lead to adverse reactions. The usual dose is 10 mg/kg on day 1, followed by two doses of 5 mg/kg 24 hours apart. This dosage, which was based on a small (n = 34) phase I study of early (within 3 hours of birth) administration of intravenous ibuprofen lysine, has since remained the most commonly used dosage regimen in clinical and research settings irrespective of the gestational and postnatal age.51 It has been demonstrated in a dose-finding study that the standard 10-5-5 mg/kg dosing regimen had a very low probability of success in extremely preterm infants born <27 weeks of gestation (30.6%, 95% [Credible intervals] CrIs 13–56%).52 Pharmacokinetic studies have also suggested that to achieve therapeutic serum concentrations, higher doses may be required with increasing postnatal age (15-7.5-7.5 mg/kg for postnatal ages of 3–5 days and 20-10-10 mg/kg for postnatal ages >5 days), further emphasizing the importance of postnatal age–dependent dosing.43 This could partly explain why the efficacy of ibuprofen for PDA closure (around 71–74%) demonstrated in RCTs of early therapy (median age of start of ibuprofen: 3 days) have not been replicated in clinical practice where therapy is usually delayed (median age 6 days) with a primary pharmacotherapy failure rate of around 40–50%.53,54 In fact, meta-analyses of RCTs demonstrate a reduced rate of failure to close the PDA with high doses of ibuprofen compared with standard doses (RR 0.37, 95% CI 0.22–0.61).55 Adaptive dosing in the form of continued doses of ibuprofen (up to 6 doses if PDA was not closed) was associated with a 88% closure rate (similar to indomethacin).56 Doubling of the doses during the second course was associated with 60% closure rates compared to 10% in infants receiving the same dose when a consecutive treatment protocol was used, underscoring the need for further studies on ibuprofen dosing, pharmacokinetics, and pharmacodynamics.57 Ibuprofen can be given orally or intravenously. A comparison of oral and intravenous ibuprofen pharmacokinetics demonstrated that the peak serum levels are reached earlier with intravenous delivery; however, the elimination is slower after enteral administration, resulting in a higher area under the curve (AUC) for serum ibuprofen concentration with the latter (AUC0-24, 618 μg/mL·h for enteral vs. 462 μg/mL·h for intravenous).58 Several trials have also compared the efficacy of routes of administration. A systematic review of oral versus intravenous ibuprofen indicated a lower risk of failure to close a PDA with oral ibuprofen use (RR 0.38, 95% CI 0.26–0.56).55 Furthermore, higher rates of sustained closure have been observed after continuous infusion compared to bolus infusion (closure after one or two courses 86% in continuous infusion group versus 68% after one or two courses in intermittent infusion group; P = 0.02).59 Intravenous ibuprofen is available in two preparations, ibuprofen lysine and ibuprofen-tris-hydroxymethyl-aminomethane (THAM). In one retrospective study from Italy it was identified that ibuprofen lysine was more effective than ibuprofen THAM in reducing the need for PDA ligation (73% vs. 51%, P = 0.002) when used prophylactically in neonates of ≤28 weeks’ gestation.60 There is ongoing controversy on whether the efficacy significantly varies between the two formulations. A systematic review and network meta-analysis of existing RCTs is currently underway to address this question.61 Similar to indomethacin, ibuprofen is also an effective agent for closure of the PDA. The response rate for the first and second courses mimics that of indomethacin, with approximate closure rates of 70% and 50%, respectively. A detailed systematic review of studies evaluating the therapeutic use of ibuprofen revealed that both oral (RR 0.26, 95% CI 0.11–0.62) and intravenous (RR 0.62, 95% CI 0.44–0.86) ibuprofen reduces failure of PDA closure in comparison to placebo treatment.55 Ibuprofen has been compared to indomethacin in several RCTs. A systematic review of these trials indicated that ibuprofen and indomethacin (both oral and intravenous) are similar in terms of their efficacy in ductal closure (RR 1.07, 95% CI 0.92–1.24), rates of PDA reopening after the first course (RR 1.57, 95% CI 0.83, 2.99), and need for surgical ligation (RR 1.06, 95% CI 0.81, 1.39).55 In addition, ibuprofen was associated with a lower incidence of renal dysfunction (as indicated by its effect on urine output and serum creatinine), shorter duration of mechanical ventilation (−2.4 days, 95% CI −3.7 days to −1.0 day), and lower incidence of NEC (RR 0.68, 95% CI 0.49–0.94). There were no differences in P/IVH, ROP, and survival or neurodevelopmental outcomes between the two agents.55 Similar to indomethacin, ibuprofen has also been used for prophylactic, asymptomatic, and symptomatic PDA management. Prophylactic ibuprofen may marginally reduce the risk of severe P/IVH (RR 0.67, 95% CI 0.45–1.00).62 Use of prophylactic oral or intravenous ibuprofen reduces the incidence of PDA on postnatal day 3 or 4 (RR 0.39, 95% CI 0.31–0.48), need for rescue treatment with cyclooxygenase inhibitors (RR 0.17, 95% CI 0.11–0.26), and need for surgical PDA ligation (RR 0.46, 95% CI 0.22–0.96) compared with placebo or no treatment, but it has no benefit on any other patient-important clinical outcomes.62 Yoo et al. evaluated mortality and neonatal complications following the use of ibuprofen in two groups of neonates, including 14 (15.4%) preterm infants of <28 weeks’ gestation with clinical symptoms of hsPDA and 77 (84.6%) asymptomatic neonates with no evidence of hsPDA.63 Infants in the symptomatic group were of younger gestation (by 1 week) and lower birth weight (by 225 g) and had higher severity of illness scores. They also received more courses of ibuprofen. In a logistic regression analysis after adjustment for severity of illness, birth weight, birth year, and invasive ventilator care ≤2 postnatal days, there were no significant differences in mortality, frequency of secondary ligation, NEC, P/IVH, BPD, or death between the two groups. The authors concluded that treatment of asymptomatic or non-hsPDA may not be warranted. A recent systematic review on early treatment versus expectant management of the hemodynamically significant PDA showed that very early treatment with ibuprofen (defined as treatment initiated <3 days of age) may reduce the incidence of BPD (RR 0.54, 95% CI 0.35–0.83).43 The review failed to demonstrate benefit of early (defined as treatment initiated <7 days) or very early therapy for any other clinical outcomes, while both approaches were associated with increased exposure to NSAIDs compared to expectant management.43 Major side effects of ibuprofen include oliguria, high bilirubin levels, gastrointestinal hemorrhage, and pulmonary hypertension. The use of ibuprofen-THAM has been associated with higher rates of gastrointestinal complications, as well as pulmonary hypertension.64 The association of ibuprofen use with pulmonary hypertension was initially thought to be specific with the ibuprofen-THAM preparation, possibly related to acidification of ibuprofen solution by the use of normal saline flush, subsequently causing precipitation and embolism.65 However, there are similar reports of pulmonary hypertension with early use of ibuprofen lysine preparation as well.66,67 In a recent cohort of 144 neonates who received ibuprofen treatment for PDA, 10 cases developed pulmonary arterial hypertension, of which 7 occurred in the intravenous ibuprofen-THAM group (n = 100), 2 in the oral ibuprofen group (n = 40), and 1 in those who received intravenous ibuprofen lysine preparation (n = 4).66 Risk factors for the development of pulmonary arterial hypertension were noted to be small for gestational age, maternal hypertension, and oligohydramnios.66 Several studies have confirmed that ibuprofen has similar potency for PDA closure as indomethacin but carries a lower profile of side effects. Therefore ibuprofen should be considered the pharmacotherapy of choice for a symptomatic PDA over indomethacin. Higher-dose ibuprofen may be considered, especially for preterm infants beyond the first 3–5 days of age. However, caution should be exercised when treating extremely preterm infants with high-dose ibuprofen due to limited safety and efficacy data. Given that oral ibuprofen is as effective as intravenous ibuprofen and is likely to result in reduced side effects, it may be the preferred route for ductal closure in infants who are receiving enteral feeds. Acetaminophen, also known as paracetamol, acts on PDA closure through the inhibition of POX-mediated conversion of prostaglandin G2 to prostaglandin H2. In addition, there is some evidence that acetaminophen may have a substantial inhibitory effect on the COX-2 enzyme.68 There has been a growing interest in the use of acetaminophen as a potential pharmacotherapy for PDA in extremely preterm infants given its relatively better safety profile compared to indomethacin or ibuprofen. The compound’s pharmacokinetics, including the dose and route of administration, have not been well-studied compared to indomethacin and ibuprofen. The potential effect of acetaminophen in closing the PDA was first reported in a case series of 5 preterm infants (GA 26–32 weeks; postnatal age 3–35 days) where the index case was administered oral acetaminophen at a dose of 15 mg/kg per dose every 6 hours orally for 7 days incidentally for an unrelated reason.69 Since then, the same dosage of 15 mg/kg dose every 6 hours for 2–7 days has been used in clinical trials and clinical practice with little exploration of dose-dependent effects.69,70 A recent study that explored the pharmacokinetic profile of acetaminophen administered at a dose of 20 mg/kg loading within 24 hours of birth followed by 7.5 mg/kg every 6 hours for 4 days in preterm infants born <32 weeks’ gestation as a part of the Preterm Infants’ Paracetamol Study (PreParaS trial) demonstrated that the efficacy of acetaminophen for ductal closure was substantially reduced with each week of gestation below 27 weeks. This highlights the need for optimal dose finding studies, especially in extremely preterm infants at the highest risk of PDA-attributable morbidity.71 The most commonly used route of acetaminophen administration is oral. The increased availability of the intravenous formulation presents an attractive option for clinicians, especially for preterm infants who are unable to tolerate feeds and have contraindications to NSAIDs. However, an RCT of 86 VLBW infants showed that indomethacin was more effective in closing a PDA (55% vs. 6%) and preventing procedural closure (15% vs. 47%) versus intravenous acetaminophen.72 Another RCT of 101 VLBW infants demonstrated that standard dose ibuprofen was more effective in closing a PDA (78% vs. 52%; P = 0.026) as compared to intravenous acetaminophen. These findings call into question the efficacy of the intravenous formulation in the highest-risk population of very preterm infants.73 There have been 27 published RCTs on the use of acetaminophen for treatment of PDA. These include comparisons with placebo, indomethacin, and ibuprofen, as well as combination therapy with ibuprofen.74 In addition, 24 ongoing trials have been identified, which are expected to add to the current body of evidence. The overall efficacy of acetaminophen is approximately 70% when compared to both ibuprofen (18 RCTs, 1535 infants) and indomethacin (4 RCTs, 380 infants). There was moderate certainty of evidence to suggest that acetaminophen is as effective as ibuprofen and low certainty of evidence to suggest that acetaminophen is as effective as indomethacin in closing a PDA.74 There were no differences in any other patient-important clinical outcomes with acetaminophen when compared with ibuprofen or indomethacin. Efficacy in the subgroup of infants <32 weeks’ gestation ranged from 67 to 76%.74 However, there remains paucity of data in the extremely preterm population born <28 weeks’ gestation. Combination therapy using acetaminophen and ibuprofen has been explored with variable success.75 Meta-analysis of two small RCTs (111 infants) failed to demonstrate any significant difference in failure of PDA closure when compared to ibuprofen alone (RR 0.77, 95% CI 0.43–1.36).74 However, failure of PDA closure may be marginally lower after two courses of combination therapy compared to ibuprofen monotherapy (RR 0.28, 95% CI 0.08–0.99). Given the relative safety of acetaminophen, this is an area that will benefit from future large trials. Most clinical trials have explored the use of acetaminophen for symptomatic PDA treatment; few have explored its prophylactic use. A meta-analysis of three RCTs showed that although prophylactic acetaminophen was effective in closing a PDA (RR 3.70, 95% CI 2.38–5.56), this higher efficacy failed to translate into a significant improvement for any other clinical outcome.74 Given the paucity of good-quality pharmacokinetic data, it is uncertain if acetaminophen efficacy is a function of postnatal age, similar to ibuprofen, and whether dose adjustments are required for later treatment. A recent systematic review suggests early acetaminophen use (initiated within 14 days after birth) was associated with a large reduction in failure to close a PDA (2 RCTs, 127 infants, RR 0.35, 95% CI 0.23–0.53), while a similar effect was not observed with later initiation of acetaminophen (1 RCT, 55 infants, RR 0.85, 95% CI 0.72–1.01).74 However, given the better safety profile, some clinicians may still choose to use a third course of pharmacotherapy using acetaminophen, following two initial failed courses of NSAIDs, while contemplating procedural PDA closure. This practice requires further interrogation as a recent retrospective observational study did show that extremely preterm infants with a persistent symptomatic PDA who were treated with a 3- to 7-day course of oral acetaminophen following two failed courses with indomethacin or ibuprofen had reduced rates of surgical ligation but increased rates of CLD.76 Acetaminophen is generally well tolerated, with substantially lower short-term adverse effects as compared to indomethacin and ibuprofen. A meta-analysis of RCTs suggests acetaminophen is associated with lower rates of NEC (4 RCTs, 384 infants, RR 0.42, 95% CI 0.19–0.96) and gastrointestinal bleeds (7 RCTs, 693 infants, RR 0.37, 95% CI 0.19–0.73) as compared to indomethacin and ibuprofen, respectively.74 Other side effects may include hepatotoxicity, as well as hemodynamic and thermodynamic effects. Therapeutic doses of acetaminophen used for analgesic purposes have not been shown to be associated with acute side effects. The oral preparation of acetaminophen is often diluted significantly prior to administration due to concerns about the solution’s high osmolality and the potential associated risk for the subsequent development of NEC. There is limited data on the effect of acetaminophen on neurodevelopmental outcomes. Viberg et al77 showed that exposure to acetaminophen during a critical period of brain development can induce long-lasting effects on cognitive function in mice and alter the adult response to acetaminophen. Moreover, acute neonatal exposure in mice also led to altered locomotor activity and affected spatial learning in adulthood. Avella-Garcia et al.78 followed children of 1 year and 5 years of age who were exposed to maternal intake of acetaminophen before 32 weeks’ gestation. The authors found that prenatal exposure to acetaminophen may alter attention function at 5 years of age while affecting males and females differently. However, this study suffers from issues of inexact delineation of the quantity, timing, and duration of maternal acetaminophen intake. Through analyzing data from a population-based cohort in Denmark, Liew et al.79 reported that prenatal use of acetaminophen was associated with a higher risk of hyperkinetic disorder (hazard ratio 1.37; 95% CI 1.19–1.59), need for attention deficit hyperactivity disorder treatment (hazard ratio 1.29; 95% CI 1.15–1.44), and attention deficit hyperactivity disorder–like behaviors at 7 years of age (RR 1.13; 95% CI 1.01–1.27). There was also some evidence for a dose-response relationship. Finally, in an ecological analysis Bauer and Kriebel80 reported that country-level autism was correlated with rates of circumcision. Given that this procedure is often accompanied by the use of acetaminophen for pain management, the possibility of an associative link has been entertained. Further data on both the side effects and the impact of acetaminophen on neurodevelopmental outcomes are clearly needed. Acetaminophen appears to be as effective as ibuprofen and indomethacin in closing a PDA while having a favorable short-term safety profile. Future efforts should address issues around optimal dosage (as monotherapy or in conjunction with other NSAIDs), route, duration of therapy, and its effectiveness in extremely preterm neonates, who are the most likely candidates for treatment. In addition, potential long-term effects of neonatal acetaminophen administration on neurodevelopment need to be examined. All three agents have been tested against one another in various combinations (Figure 17.1) with no major differences observed for any of the patient and family important clinical outcomes, with the exception of the effect of prophylactic indomethacin on decreasing the rates of P/IVH. There is currently no RCT that has evaluated all the three agents (acetaminophen, ibuprofen, and indomethacin) as prophylactic therapies in the same trial. A recent Bayesian network meta-analysis (28 RCTs, 3999 infants) evaluated the comparative effectiveness and safety of all prophylactic therapies versus ’no prophylaxis’ in preterm infants.81 The authors concluded that prophylactic indomethacin probably results in a small reduction in severe IVH (network RR 0.66, 95% credible intervals [CrI] 0.49 to 0.87; absolute risk difference [ARD] 43 fewer [95% CrI, 65 fewer to 16 fewer] per 1000; median rank 2; moderate certainty), a moderate reduction in mortality (network RR 0.85, 95% CrI 0.64–1.1; ARD 24 fewer [95% CrI, 58 fewer to 16 more] per 1000; median rank 2; moderate certainty), and surgical PDA closure (network RR 0.40, 95% CrI 0.14–0.66; ARD 52 fewer [95% CrI, 75 fewer to 30 fewer] per 1000; median rank 2; moderate certainty), but it may result in a small increase in CLD (network RR 1.10, 95% CrI 0.93–1.3; ARD 36 more [95% CrI, 25 fewer to 108 more] per 1000; low certainty). Prophylactic ibuprofen probably results in a small reduction in severe IVH (network RR 0.69, 95% CrI 0.41–1.14; ARD 39 fewer [95% CrI, 75 fewer to 18 more] per 1000; median rank 2; moderate certainty) and moderate reduction in surgical PDA closure (network RR 0.24, 95% CrI 0.06–0.64; ARD 66 fewer [95% CrI, from 82 fewer to 31 fewer] per 1000; median rank 1; moderate certainty) and may result in a moderate reduction in mortality (network RR 0.83, 95% CrI 0.57–1.2; ARD 27 fewer [95% CrI, from 69 fewer to 32 more] per 1000; median rank 2; low certainty). For acetaminophen, the estimate of effects was very uncertain for any of the clinically relevant outcomes. With regards to management of symptomatic PDA, to date, only one randomized study has evaluated all three agents (acetaminophen, ibuprofen, and indomethacin) relative to each other.82 In this prospective study preterm neonates, born at a gestational age less than 28 weeks’ or weight of <1500 g, who were diagnosed with hsPDA by echocardiography and clinical examination were randomized to one of the three intervention groups (100 in each arm). Patients received a second course of the same treatment regimen if the PDA failed to close. Overall, there was no difference in the rate of ductal closure after the first (80% vs. 77% vs. 81%) or second course (88% vs. 83% vs. 87%) of acetaminophen, ibuprofen, and indomethacin, respectively. There was also no difference in neonatal complications and side effects between all groups, with the exception of a higher occurrence of intestinal bleeding in the indomethacin- and ibuprofen-treated patients (P < 0.05).82 A Bayesian network meta-analysis of 68 RCTs (4802 infants) showed that high-dose oral ibuprofen was associated with a significantly higher odds of PDA closure versus intravenous formulations of standard dose ibuprofen (OR 3.59; 95% CrI 1.64–8.17) or indomethacin (OR 2.35; 95% CrI 1.08–5.31).20 However, data was insufficient to draw meaningful conclusions on clinically important outcomes such as CLD, NEC, and IVH. Despite a large body of evidence from RCTs, pharmacotherapy of the PDA in preterm infants remains controversial. In summary, ibuprofen has replaced indomethacin as the gold standard in infants where medical management is sought, whereas acetaminophen has emerged as a potentially less toxic alternative, with similar efficacy to the NSAIDs. Clinicians are increasingly considering the use of higher doses of ibuprofen, preferably using enteral formulations, especially for delayed treatment.19 However, the safety of such approaches in the most vulnerable preterm population is yet to be firmly established. From a pharmacoprophylaxis perspective, clinicians have generally moved away from using NSAIDs in all extremely preterm infants. However, with increased survival of infants at the limits of viability, some clinicians have reconsidered the use of selective pharmacoprophylaxis using indomethacin, especially in infants at the highest risk of P/IVH and mortality, while there is a growing interest in exploring the use of prophylactic or early selective use of acetaminophen in extremely-low-gestational-age infants given its favorable short term safety profile. The effects of such approaches on long-term neurodevelopmental, cardiovascular, and renal health are yet to be determined. It is important to recognize that most trials of PDA pharmacotherapy were designed to examine whether pharmacotherapy was effective in closing a PDA. Most of the RCTs were not designed or powered to explore whether pharmacotherapy for PDA improved clinically relevant outcomes. Hence most trials had a backup treatment option had the PDA remained open following the initial intervention, as evidenced by the significantly higher rate of open-label treatment in the placebo group.83 Therefore caution should be exercised while assessing the benefits and harms of pharmacotherapy in light of patient-important outcomes. Further, with regard to patient-important outcomes, it is important to highlight the over-emphasis on BPD as the primary clinical endpoint in many of the previous trials. BPD, commonly defined in previous RCTs as oxygen requirement at 36 weeks postmenstrual age, may not be the most important clinical outcome from a parent’s perspective, as compared to other respiratory morbidities, such as need for mechanical ventilation at 36 weeks postmenstrual age (severe BPD) or chronic severe pulmonary hypertension. Therefore future trials should be adequately powered for outcomes that are clinically meaningful for patients and their families. The diagnosis of a hemodynamically significant PDA remains a challenge in clinical trials. Although echocardiography characteristics have been identified, they are not reliable enough to effectively identify the right population who would benefit from early effective closure of the ductus (these concepts are further discussed in Chapters 16 and 19). Even in cases where perturbation of blood flow to splanchnic organs has been identified, its clinical significance remains uncertain. This has led to a growing skepticism around the management of ductus in preterm infants, with some clinicians/centers advocating in favor of abandoning PDA evaluation and treatment altogether. To better guide clinicians in the management of PDA, further understanding of the physiologic changes caused by the condition is required. The identification of biochemical or imaging biomarkers that signal affected organ blood flow arising from the systematic diversion of blood to the lungs by a significant PDA may complement current diagnostic tools. Furthermore, the identification of mechanisms by which higher pulmonary vascular flow secondary to hsPDA affects the developing lungs and pulmonary blood flow regulation may help guide the development of new therapies, as not all neonates with higher flow result in pulmonary edema and hemorrhage.84 Pharmacokinetics and pharmacodynamics of all three agents are poorly studied and future studies should build that component in their design so that such questions can be answered simultaneously rather than as afterthoughts. Ideally, randomized controlled studies would be the preferred approach to evaluate the impact of diagnostic tests or therapeutic interventions. However, large cohorts could also be studied with careful selection of analytical techniques that incorporate as many residual confounding variables as possible (propensity score methods, instrumental variable analyses) and answer two critical biases associated with the assessment of long-term outcomes of PDA, confounding by indication or contraindication and survival bias.85 Future studies will need to try to account for these biases when assessing the impact of therapies on survival free of neurodevelopmental impairment. In the meantime, clinicians will continue debating the risks and benefits of various treatment options until enough evidence is available to establish generally accepted guidelines for the management of PDA in the preterm neonate. Finally, utilizing comprehensive, real-time monitoring and data acquisition systems along with mathematical modeling and machine learning (Chapter 14), differences among individual patients affecting PDA rates, rates of spontaneous closure, and the patient’s response to the different treatment approaches could also be recognized and modeled to the phenotypic profile of the given patient, providing a precision medicine approach to the pharmacological management of the PDA.
Chapter 17: Pharmacological management of patent ductus arteriosus in the very preterm neonate
Introduction
Epidemiology of PDA treatment
Pharmacological interventions
Indomethacin
Mechanism of action
Dose, route, and frequency
Efficacy
Timing of administration
Side effects
Take-home message
Ibuprofen
Mechanism of action
Dose, route, and frequency
Efficacy
Timing of administration
Side effects
Take-home message
Acetaminophen (paracetamol)
Mechanism of action
Dose, route, and frequency
Efficacy
Timing of administration
Side effects
Take-home message
Comparison of the three pharmacological agents
Conclusions and implications for practice and research
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree