DIAGNOSTIC AND THERAPEUTIC MODALITIES
JAMES J. BURKE II  SCOTT C. PURINTON
SCOTT C. PURINTON  HEATHER MACNEW
HEATHER MACNEW
INTRODUCTION
Surgery remains the mainstay of treatment for women with gynecologic malignancies. Ultimately, outcomes of the surgical intervention rest with the gynecologic oncologist in concert with anesthesiologists, nursing staff, stomal therapists, physical therapists, pharmacists, social workers, and the social network/support of the patient as well as others. Careful assessment of the patient prior to surgery can lead to improved outcomes and minimize surprises in the postoperative period. Should the need arise, prudent consultation with other medical specialists prior to or following surgery can further enhance patient care, and result in better outcomes.
The chapter has been divided into (2) sections: preoperative care/risk recognition and postoperative care/critical care. Within each section, clinical information has been arranged by organ system and recommendations are based upon evidence (when available). The critical care section provides basic yet practical information for the reader so that comanagement of the critically ill gynecologic oncology patient with an intensivist may be seamless.
PREOPERATIVE RISK ASSESSMENT
Initial Preoperative Evaluation
At the initial consultation for patients with known or suspected gynecologic malignancies, the gynecologic oncologist should take a thorough history, assessing for comorbid conditions, which may impact perioperative risk (1). Similarly, a thorough physical examination, looking for signs of diseases of which the patient is unaware, will aid in finding diseases that can impact surgical outcome. Review of accompanying medical records and radiographs is important. Ultimately, patients who will benefit from surgery are identified and will be deemed operative candidates, operative candidates who need further evaluation from specialists prior to surgery, or inoperable candidates.
Subsequent discussions should focus on the course of treatment. If surgical, the planned operative procedure should be described to the patient in nonmedical terminology. Attendant risks of the procedure, as well as alternatives for therapy (if they exist), should be described to the patient. The length of time for the operation and length of anticipated hospital stay should be estimated for the patient and her family. Further, time to recovery from the planned operative procedure should be estimated for the patient. These elements of the treatment plan constitute informed consent, and should be documented in the medical record by the physician at the initial consultation. Preferably, this “consenting” should be done before the patient is in the preoperative holding area on the day of her surgery. Should further evaluation be needed from a specialist (e.g., a cardiologist or a pulmonologist), a letter outlining the proposed surgical intervention should be sent to the consultant. However, the impact of these consultations, or “preoperative clearances,” on perioperative outcomes is unclear (2 – 4).
Ideally, preoperative laboratory testing will be dictated by findings from the history and physical examination. Unfortunately, unnecessary and inappropriate preoperative testing has been done in an effort to reduce poor perioperative outcomes. However, evidence to support this practice is lacking and the cost to complete these unnecessary tests has been estimated to be over $3 billion (5). In order to standardize preoperative testing, evidence-based guidelines have been developed (6). The National Institute for Health and Clinical Excellence (NICE), an independent organization in the United Kingdom that produces evidence-based guidelines for the promotion of good health and treatment of disease, developed guidelines for preoperative testing. These guidelines take into account the patient’s age, type of surgery, associated comorbidities, and the American Society of Anesthesiologists (ASA) grade for anesthesia risk (7) (Table 8.1). St. Clair et al. performed a retrospective study assessing adherence to the NICE guidelines for preoperative testing in patients having gynecological surgery between 2005 and 2007. The authors found that among 1,402 patients evaluated, inappropriate preoperative testing resulted in costs over $418,000 (8).
If the patient’s condition requires the possibility of a stoma(s) (colostomy or urostomy), consultation with an enterostomal therapist for marking of the planned stoma(s) should be considered. During this visit, the therapist will take into account the location of the patient’s waist, abdominal creases, how she wears her clothing, the types of clothing she wears, and the location of the future stoma when she stands or sits. In addition, the therapist can initiate education on the function and care of the stoma(s).
Should the proposed surgery result in a marked change of body image or possible sexual dysfunction (e.g., exenteration, radical vulvectomy, or vaginal reconstruction), consultation with prior patients who have successfully recovered from similar operations may be warranted. In addition, these patients may benefit from psychological counseling prior to their surgery.
Assessment of Cardiac Risk
Any gynecologic oncologist must be aware of the underestimation of cardiac disease in women when evaluating cardiac risk preoperatively. In the past decade, a great deal of literature has been published on this subject. In 2001, the National Heart, Lung, and Blood Institute (NHLBI) launched the Heart Truth Project to promote education about heart disease among women (9). Statistics have shown that only one of three primary physicians correctly cited coronary artery disease as a leading cause of death in women. Similarly, studies have demonstrated that women are less often counseled on cardiac risk factors, less often prescribed lipid-lowering medications, less often offered invasive procedures, and less often prescribed cardiac rehabilitation. There is a lack of health care provider knowledge of the guidelines for prevention of cardiovascular disease (CVD) in women (10). Further, compared to men, women who had a myocardial infarction (MI) had a greater interval from onset of pain until arrival at hospital, were less likely to be treated with thrombolytics and β-blocking agents, were less often evaluated with invasive methods, had higher rates of reinfarction, and had greater mortality (9).
National Institute for Health and Clinical Excellence Recommendations for Preoperative Testing |
Study | Age (years) | Recommendation |
Chest X-ray | Any age | Cardiovascular surgery |
| 60 or older | Grade 4 surgery and ASA 3 or greater with cardiovascular disease |
Electrocardiogram | 16–39 | ASA 2 or greater with cardiovascular disease or cardiovascular surgery |
| 40–59 | As above, plus grade 4 surgery if ASA 2 or greater with renal disease, or ASA 3 with respiratory disease |
| 60–79 | As above, plus grade 2 surgery if ASA 2 or greater with renal disease, or ASA 3+ with respiratory disease or grade 3 surgery or greater |
| 80 or older | Consider deferring if grade 1 surgery |
Full blood count | 16–59 | ASA 3 with renal disease, or grade 3 or greater surgery |
| 60 or older | As above, plus grade 2 or greater surgery |
Hemostasis | 16 or older | Never recommend, may be considered |
Renal function | 15–59 | ASA 2 or greater with renal disease, ASA 3 or greater with cardiovascular disease, ASA 2 or greater with cardiovascular disease with grade 3 or greater surgery, ASA 3 or greater with respiratory disease, with grade 3 or greater surgery |
| 60 or older | As above, plus ASA 2 with cardiovascular disease, with grade 2 or greater surgery, or any grade 3 surgery |
Urinalysis | 16 or older | Never recommended, may be considered |
ASA, American Society of Anesthesiologists.
ASA grade: ASA 1, normal/healthy; ASA 2, mild systemic disease; ASA 3 severe systemic disease.
Surgery grade: grade 1, minor surgery; grade 2, intermediate surgery; grade 3, major surgery; grade 4, extensive surgery.
Source: From St. Clair CM, Shah M, Diver EJ, et al. Adherence to evidence-based guidelines for preoperative testing in women undergoing gynecologic surgery. Obstet Gynecol. 2010;116(3):694–700, with permission.
Multifactorial Index of Cardiac Risk |
Risk factor | Points |
S3 gallop or increased jugular venous pressure | 11 |
Myocardial infarction in previous 6 months | 10 |
More than 5 premature ventricular ectopic beats per minute | 7 |
Rhythm other than sinus or premature atrial contractions | 7 |
Age >70 years | 5 |
Emergency noncardiac operative procedure | 4 |
Significant aortic stenosis | 3 |
Poor general health status | 3 |
Abdominal or thoracic surgery | 3 |
Possible total | 53 |
Source: Adapted from Goldman L, Caldera DL, Nussbaum SR, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med.
1977;297:845–880, with permission.
In the assessment of perioperative risk, cardiac risk factors are certainly one of the top concerns for clinicians. There have been a number of reviews and different systems created for the purposes of assessing cardiac risk for patients undergoing noncardiac surgery (10–13). Realize that approximately 1 in 12 patients (>65 years old) will have significant coronary artery disease (14). It is estimated that over 30% of patients undergoing major elective surgery have at least one cardiac risk factor (15).
Cardiac risk indices have been published by at least 10 different investigators (16). Goldman et al. published the Multifactorial Index of Cardiac Risk (MICR) in 1977 (17). This risk index was the first large, prospective, multivariate analysis of patients undergoing noncardiac surgery. They used definite endpoints of cardiac death, ventricular tachycardia, pulmonary edema, and myocardial infarction. The MICR involves nine independent risk factors to create a point risk index and predict morbidity and mortality (Tables 8.2 and 8.3). One weakness of this index is underestimating risk in vascular surgery patients. Nonetheless, these criteria have been validated and have stood the test of time. In response to a shift in the literature from calculation of risk with indices to clinical decision making, especially in regard to the need for preoperative evaluation, the American College of Cardiology/American Heart Association (ACC-AHA) guidelines were developed (18). Recently, the AHA updated guidelines for CVD prevention in women (19). This document provides risk classification of coronary vascular disease (CVD), based upon clinical criteria and/or the Framingham 10-year global risk score (20) (Table 8.4). This CVD risk stratification has not been assessed specifically for preoperative risk assessment, but provides classification of women who may need further (noninvasive or invasive) evaluation.
Multifactorial Index of Cardiac Risk, Cardiac Risk Class, Morbidity, and Mortality |
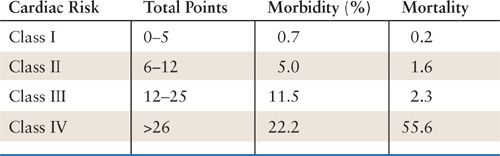
Source: Adapted from Goldman L, Caldera DL, Nussbaum SR, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297:845–850, with permission.
Classification of CVD Risk in Women |
Risk Status | Criteria |
High risk | Established coronary heart disease |
| Cerebrovascular disease |
| Peripheral arterial disease |
| Abdominal aortic aneurysm |
| End-stage or chronic renal disease |
| Diabetes mellitus |
| 10-year Framingham global risk >20%a |
At risk | ≥1 major risk factors for CVD, including: |
| Cigarette smoking |
| Poor diet |
| Physical inactivity |
| Obesity, especially central adiposity |
| Family history of premature CVD (CVD at <55 years of age in male relative and <65 years of age in female relative) |
| Evidence of subclinical vascular disease (e.g., coronary calcification) |
| Metabolic syndrome |
| Poor exercise capacity on treadmill test and/or abnormal heart rate recovery after stopping exercise |
Optimal risk | Framingham global risk <10% and a healthy lifestyle, with no risk factors |
CVD, coronary vascular disease.
aOR at high risk on the basis of another population-adapted tool used to assess global risk.
Source: Adapted from Mosca L, Banka CL, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation. 2007;115:1481–1501, with permission.
Clearly, the approach to the patient must include a careful history and physical examination. Initially, age greater than 70 years was thought to be a risk factor for cardiac morbidity, but a recent clinical trial showed no increased independent risk for cardiac complications (20). Any prior history of cardiac disease such as angina, MI, arrhythmia, congestive heart failure, or valvular disease must be evaluated. Patients with unstable angina, recent myocardial infarction, class III-IV heart failure, decompensated congestive failure, or aortic stenosis present the highest risk. These patients will likely require further invasive testing. Severe aortic stenosis must be identified preoperatively because the risk of perioperative morbidity is as high as 30% (21).
In patients without overt cardiac risks, other factors are considered to be helpful for uncovering subclinical disease. Classically, these risk factors are smoking, hyperlipidemia, hypertension, and diabetes mellitus.
In 2007, the ACC-AHA published revised guidelines to direct invasive, interventional evaluations (14). The classification defines clinical predictors as minor, intermediate, and major. In addition, the guidelines now utilize functional capacity in terms of metabolic equivalents (METs), with a level <4 being considered poor. The ability to climb one flight of stairs or walk up a hill would classify the patient in the >4 group (14).
Testing available for further evaluation includes both invasive and noninvasive methods. The routine resting electrocardiogram is a valuable screening tool for patients over the age of 40. It can potentially identify a prior myocardial infarction, which may prompt further evaluation of coronary artery disease. Echocardiography can predict postoperative congestive heart failure (CHF) in patients with ejection fractions (EFs) less than 35% (22). Unfortunately, echocardiography cannot reliably predict ischemia, but may be quite useful in the evaluation of valvular diseases and for follow-up of patients with known left ventricular (LV) dysfunction. Additionally, elevated preoperative brain natriuretic peptide (BNP) is an independent predictor of 30-day adverse cardiovascular outcomes after noncardiac surgery (23). Exercise or pharmacological stress testing provides valuable information for perioperative ischemic risk. Nuclear scintigraphy with evaluation of perfusion defects has shown a positive predictive value of 12% to 16% and a negative predictive value of 99% (24). Dobutamine stress echocardiography has shown similar predictive values.
The ACC-AHA recommendations provide a way to segregate patients who should have their surgery delayed for further cardiac evaluation because of a recent MI, who should have their CHF optimized, or who should optimize control of dysrhythmias. In selected patients, coronary revascularization, angioplasty, stent placement, or valve replacement may be prudent before the planned noncardiac surgery (18).
The risk of reinfarction after a recent MI is directly related to the interval between the MI and an event, which could precipitate an MI. However, these rates have been declining due to improved perioperative care. Reinfarction rates have dropped from 37% in patients undergoing noncardiac surgery within 3 months following MI to 5% to 10%, more recently. Reinfarction rates reduce further as the interval from the original MI increases, with rates of reinfarction being 2% to 3% and 1% to 2%, 4 to 6 months and greater than 6 months, following the acute event, respectively (25). Elective surgery should be postponed for 6 months following an acute MI, however an urgent operation may be performed 4 to 6 weeks after infarction if stress testing is favorable and the patient receives cardiac monitoring in the perioperative period.
Perioperative β-Blockade
In 1996, a multicenter, randomized, placebo-controlled trial was published that evaluated the use of β-blockade with atenolol versus placebo in patients undergoing noncardiac surgery. Although no differences in perioperative mortality or MI were seen, the atenolol group had significantly fewer ischemic episodes (24% vs. 39%). Furthermore, at 6 months and at 2 years, the atenolol group had decreased mortality (9 vs. 21 deaths) and decreased number of cardiac events (16 vs. 32) (26). The Peri Operative ISchemic Evaluation (POISE) trial showed that the use of β-blockers in the perioperative period reduces the risk of the composite outcome of cardiovascular death, nonfatal myocardial infarction, and nonfatal cardiac arrest at 30 days. However, it also shown that bradycardic episodes and hypotensive events result in an increase in death and strokes (27). Based on the findings of these studies, patients with or at risk for coronary artery disease may receive some benefit from perioperative β-blockade use; however, the clinician must be aware of the potential adverse outcomes.
PULMONARY RISK ASSESSMENT
Postoperative pulmonary complications represent a significant cause for morbidity and mortality in patients undergoing elective surgery. Approximately 25% of deaths within the early postoperative period (first week) are related to pulmonary issues. Pulmonary complications after major abdominal surgery range from 20% to 30% (28). Laparotomy results in a 45% decrease in vital capacity and a 20% reduction in functional residual capacity (FRC) (29). When the patient is in the supine position, FRC is reduced below alveolar closing volume (i.e., the volume at which point alveoli start closing), which results in atelectasis (30).
When examining risk factors for postoperative pulmonary problems, a number of issues surface. General medical status (e.g., functional status, obesity, nutrition) is related to postoperative pulmonary complications (PPCs) (31). A history of congestive heart failure, renal failure, poor mental status, and immunosuppression are all associated with a higher PPC rate (32). Surgical issues such as the type of procedure (open or minimally invasive), the type of incision (thoracic and upper abdominal being worse than midline or lower abdominal), duration of anesthetic (>2 hours), the use of a nasogastric tube (increased risk), and the use of parenteral (increased risk) versus epidural (decreased risk) analgesics are all correlated with PPC incidence (33). All patients undergoing noncardiothoracic surgery should be evaluated for the presence of the following risk factors for PPCs in order to receive pre- and postoperative interventions to reduce risk: chronic obstructive pulmonary disease (COPD), congestive heart failure, American Society of Anesthesiologists (ASA) class of II or greater, functionally dependent, and age older than 60 years (34). In terms of direct pulmonary risk factors, the most common preexisting pulmonary disease is COPD (32). These patients retain carbon dioxide, have poor gas exchange, and have an increased residual volume. Smoking, history of dyspnea, pneumonia, and sleep apnea are other risk categories. Patients with asthma and other restrictive lung diseases (low forced vital capacity [FVC] with normal forced expiratory volume in the first second of expiration [FEV1]/FVC ratio) have minimal increased risk for PPCs (33).
When interpreting the usual preoperative radiographic and laboratory values, several caveats must be kept in mind. A preoperative chest radiograph in normal adults has no predictive value other than providing essential baseline data for an at-risk patient. Arterial blood gas analysis in prospective trials has not been shown to be useful in providing risk stratification. However, it is useful in providing baseline data for patients with preexisting disease such as COPD (35). A low serum albumin (<35 g/L) is a powerful marker of increased risk and should be considered in patients with one or more risk factors for PPCs (36). Preoperative pulmonary function tests (PFTs) are rarely useful for risk stratification and not routinely indicated prior to surgery. A consensus statement from the American College of Physicians in 2006 recommended that preoperative PFTs or chest radiography may be appropriate in patients with a previous diagnosis of COPD or asthma (34). Such testing may provide valuable baseline data and aid in risk stratification for patients with moderate to severe COPD who are undergoing major abdominal surgery.
Perioperative strategies for reducing risk of PPCs include lung expansion techniques, smoking cessation, and optimization of gas exchange. Although preoperative and postoperative incentive spirometery has shown mixed results in reducing the rate of pulmonary complications, it continues to be widely recommended (37), and it should be considered as a preventive strategy for any patient undergoing laparotomy. In order to maximize patient compliance, preoperative counseling and education are necessary. Clearly, COPD must be optimized with control of infection and maximizing medical regimens. Reactive airway disease should be prevented with the use of perioperative inhalation therapy such as β agonists. Steroid therapy is generally reserved for patients already utilizing them as part of their medical regimen. These steroid-dependent patients will need stress-dose steroids to prevent insufficiency (see “Adrenal Suppression” section below). Prophylactic antibiotics are not indicated in COPD patients to prevent pulmonary infections.
Smokers have significantly more postoperative complications including pneumonia, surgical-site infections, and death (38). Smoking-cessation programs have had an unclear effect on PPCs (39). Although the data consist of poorly controlled trials, it appears that short-term abstinence (<8 weeks from the time of surgery) may actually increase the complication rate (40). Abstinence for greater than 10 weeks showed complication rates similar to nonsmokers (41). Unfortunately, the long-term success rate of smoking-cessation programs is low, and in the case of malignancy, the gynecologic oncologist rarely has the opportunity to delay the operation for 8 to 10 weeks.
ENDOCRINOLOGIC RISK ASSESSMENT
Diabetes
The prevalence of diabetes has reached epidemic proportions in the United States. In 2011, the Centers for Disease Control and Prevention (CDC) reported that 25.8 million people or 8.3% of the population have diabetes (42). Diabetes accounts for the fourth leading comorbid condition among hospital discharges (43). One-fifth of surgical patients will have diabetes and people with known diabetes have a 50% risk of undergoing surgery at some point in their lifetime (44). Interestingly, one-third to one-half of patients hospitalized are unaware that they have diabetes and are currently receiving no treatment. It is only during preoperative evaluation for elective surgery or acute hospitalization that these patients will be diagnosed (45). Because of this prevalence, some authors have suggested that all surgical patients be regarded as dysglycemic until proven otherwise (46). Understanding the basic physiology of diabetes and how it impacts perioperative risk is crucial for the surgeon.
There are two types of diabetes. Type 1 diabetes occurs as a result of insulinopenia, with all type 1 diabetics being insulin dependent. In the absence of sufficient insulin, these patients develop ketoacidosis. Type 1 diabetics account for approximately 10% of all diabetics. Type 2 diabetes occurs as a result of insulin resistance and impaired insulin secretion. Type 2 diabetics may be treated with diet alone, oral hypoglycemic agents, noninsulin injectable medications, or insulin. These patients account for approximately 90% of all diabetics (47). However, approximately 30% of hospitalized patients will have a prediabetic condition consisting of impaired fasting glucose levels or impaired glucose tolerance (45). Thus, perioperative glycemic control will be dictated by known (or newly discovered) diabetic status.
When evaluating patients with known diabetes for surgery, attention should be directed toward the patient’s diabetic status (i.e., type of glucose control; number of hypoglycemic events, etc.) and the long-term complications caused by diabetes since this end organ damage can impact perioperative outcome. Most complications of diabetes are related to microvascular changes, such as diabetic retinopathy, neuropathy, nephropathy, and CVD (48). In addition to a thorough history and physical examination, preoperative studies should include an electrocardiogram to rule out a prior “silent” MI (especially in patients with diabetes for more than 10 years), serum creatinine, blood urea nitrogen, urinary analyses to assess renal function and glycosylated hemoglobin (HbA1C) to evaluate recent glycemic control. HbA1C levels reflect the level of hyperglycemia that red blood cells (RBC) have been exposed. Because the average lifespan of an RBC is 120 days, the HbA1C is an indicator of glycemic control over that period of time (47). As mentioned earlier, because of the prevalence of dysglycemia among hospitalized adults in the United States, measurement of HbA1C, as a screening for diabetes, may identify patients with undiagnosed diabetes or impaired glucose metabolism (46). HbA1C levels ≤6.5% are associated with good long-term glucose control and have been associated with decreased rates of infectious complications across a variety of surgical procedures (49,50). However, HbA1C levels >6.5% are diagnostic for diabetes and evidence of poor glycemic control in known diabetics (51,52). Ultimately, the type of diabetes, preoperative glycemic control, the extent and magnitude of the intended surgery, the elective or emergent nature of said surgery, and other comorbid medical conditions will affect the metabolic changes these patients face intra- and postoperatively (46).
Another entity which has come to light in the last decade among hospitalized patients is that of stress induced hyperglycemia (SIH). This disease is defined as an inpatient fasting glucose measurement ≥126 mg/dL or a random glucose measurement of more than 200 mg/dL, which returns to normal after discharge (44,53). A growing body of evidence suggests that SIH and diabetic hyperglycemia are different diseases (54) and SIH confers a higher risk of adverse outcomes, such as an increased risk of in-hospital mortality (52,55,56) and longer lengths of stay compared to nondiabetic or hyperglycemic diabetic patients (53).
The armamentarium available for treatment of diabetes has increased over the last decade. Type 1 diabetics will most likely be using a combination of insulin therapies for glycemic control, whereas type 2 diabetics may be on oral therapy alone, insulin therapy alone, injectable noninsulin therapy alone, or some combination therapy of all the aforementioned agents. Table 8.5 outlines these drugs and timing for stopping these medications prior to surgical procedures because of the pharmacological half-lives of these drugs (57).
The physiologic changes that diabetic patients encounter during surgery result in a hyperglycemic state. The stress of surgery increases secretion of epinephrine, norepinephrine, cortisol, and growth hormone, all of which directly antagonize insulin action (46–48). In addition, gluconeogenesis and lipolysis are increased with mobilization of glucose precursors, and a net protein catabolism ensues. Glycemic control perioperatively will depend upon the type of diabetes or impaired glucose tolerance the patient has, as well as the medications that have/have not be utilized to control such disease states. Recently the Clinical Guidelines Subcommittee of The Endocrinology Society published treatment guidelines for non-critically ill patients hospitalized and subsequently found to be hyperglycemic (known diabetes versus unknown). The authors would direct the reader to review these guidelines, but recommendations from this group, for perioperative care of patients with hyperglycemia undergoing surgical interventions, are listed in Table 8.6 (58).
There are several case-control studies that demonstrate an increased risk for adverse outcomes in patients undergoing elective noncardiac surgery who have either preoperative or postoperative hyperglycemia (59–63). Postoperative glucose levels greater than 200 mg/dL are associated with prolonged hospital stays and increased risk of postoperative complications including wound infections and cardiac arrhythmias (60–62). Although diabetic patients with vascular disease are at risk of silent postoperative MI and acute renal failure, postoperative infections (respiratory, urinary, wound infections, etc.) account for about two-thirds of all postoperative complications and 20% of all postoperative deaths among diabetics undergoing surgery (49). Hyperglycemia has been shown to impair phagocytic function and chemotaxis of granulocytes when glucose levels are higher than 250 mg/dL (64).
Management of Diabetes Medications Before Surgery in Patients Who Must Be NPO |
Medication Type | Night before Surgery | Morning of Surgery |
Oral Agents |
|
|
Sulfonyl-urea |
| Hold |
Glyburide | Give with meal |
|
Glipizide |
|
|
Glimepiride |
|
|
Metformin (contraindicated in women with creatinine levels >1.4) | Hold | Hold; can induce lactic acidosis and should be held in patients’ procedures that require IV contrast |
Thiazolidinediones | Hold | Hold; Can cause fluid retention in the postoperative period |
Rosiglitazone |
|
|
Pioglitazone |
|
|
Meglitinides | Give with meal | Hold |
Repaglinide |
|
|
Nateglinide |
|
|
α Glucosidase inhibitors | Give with meal | Hold |
Acarbose |
|
|
Miglitol |
|
|
Dipeptidal peptidase-IV inhibitor | Give with meal | Hold |
Sitagliptin |
|
|
Noninsulin Injectable |
|
|
Incretin mimetics | Give 30–60 minutes before meal | Hold |
Exenatide |
|
|
Amylin analog | Give immediately before meal | Hold |
Pramlintide |
|
|
Insulin |
|
|
Regular Insulin | Give full dose | Give half dose |
Humulin ®R |
|
|
Novolin ®R |
|
|
ReliOn ®R |
|
|
NPH Insulin | Give full dose | Give half dose |
Humulin ®N |
|
|
Novolin ®N |
|
|
ReliOn ®N |
|
|
Premixed Insulins | Give full dose | Give half dose |
Humulin ®70/30 |
|
|
Novolin ®70/30 |
|
|
Humalog ®75/25 |
|
|
Novolog ®70/30 |
|
|



