Agent (author)
RCT design (N)
Follow-up (years)
Volume injected (mL)b
Dry/improvedc
Subjects requiring multiple treatments
Durasphere [38]
Multicenter
Agent (176)
1
Agent: 4.83****
–/66 %
35 %
Control (188)
Control: 6.23****
–/66 %
36 %
Durasphere [3]
Agent (25)
2.6
Agent: 4.5
40 %/80 %
Control (21)
2.8
Control: 4.2
14 %/62 %
Coaptite [43]
Multicenter
Agent (131)
1
Agent: 2.15****
39 %/63.4 %
62 %†
Control (100)
Control: 3.39****
37 %/57 %
74 %†
Macroplastique [20]
Multicenter
Agent (122)
1
Agent: 4.6
37 %†/62 %****
52 %
Control (125)
Control: 4.6
25 %†/48 %****
58 %
In a randomized, controlled, double-blind trial comparing Durasphere to bovine collagen, Andersen [3] found there was no difference in the percentage of patients with an improvement of one or more Stamey Continence Grades between the Durasphere group (80 %) and the bovine collagen group (61.9 %, p = .205) at an average of 2.6 and 2.8 years of follow-up, respectively (Table 13.1). At this long-term follow-up, 40 % (10/25) of the Durasphere subjects and 14.3 % (3/21) of the bovine collagen subjects reported that they were dry (p = .099). Complications rates were not reported in this trial.
Although Lighter et al. reported on radiologic stability of Durasphere, with no evidence of spread beyond local confines of the pelvis at 1- and 2-year follow-up [38], there have been reports of local migration into areas lateral to the urethra and along regional lymphatic chains [49]. Foreign body reactions with associated urethral prolapse and delayed presentation of a pseudoabscess 5 years postprocedure have been reported [9].
Calcium Hydroxylapatite (Coaptite)
Coaptite is composed of spherical calcium hydroxylapatite particles, ranging from 75 to 125 mm in diameter, in a carboxymethylcellulose gel carrier [16, 43]. The gel carrier degrades over several months, and the patient’s fibroblasts infiltrate amongst the particles. Calcium hydroxylapatite is a constituent of human bone and teeth and has been used for 25 years in dental and orthopedic procedures [44]. It is radio-opaque and can be easily identified with plain film radiography and ultrasound after injection [16]. Calcium hydroxylapatite is neither immunogenic nor inflammatory and remains pliable after injection into soft tissues, lending itself to use in augmentation of the vocal cords and facial structures [5, 37]. Initial studies of its endoscopic use in the treatment of vesicoureteral reflux in pediatric subjects have shown it to be both durable and efficacious [44].
In a multicenter, prospective, randomized, single-blind trial of 296 women with SUI secondary to intrinsic sphincter deficiency, subjective improvement in urinary incontinence symptoms, as graded by the Stamey Urinary Incontinence Scale, were similar at 12-month follow-up for patients who received Coaptite compared to those who received bovine collagen (63.4 % versus 57 %, respectively, p = 0.34) ([43], Table 13.1). The cure rate (Stamey grade 0) at 12 months was also similar for both groups (39 % for Coaptite versus 37 % for Contigen, p = 0.34). A greater number of patients receiving Coaptite injections required only one injection over the first 6 months of the study (38.0 % versus 26.1 %, p = 0.034); however, most subjects required two to three injections in either group. There was no difference in the percentage of patients with transient urinary retention (41 % Coaptite versus 33 % bovine collagen), and there was less postprocedural urge incontinence in the Coaptite group (5.7 % versus 12 %, p < .05). There was one vaginal wall erosion in the Coaptite group, and one patient with dissection of the Coaptite beneath the trigonal mucosa, resulting in difficulty with visualization of one ureteral orifice cystoscopically. This patient, however, had no abnormal lab or radiographic abnormalities and had no urinary incontinence.
Serious adverse events related to Coaptite are rare. Palma et al. reported on a patient presenting with a 3 cm urethral prolapse containing macrophages surrounding the Coaptite particles 3 months after initial peri-urethral bulking with a total of 2.5 mL of Coaptite [48]. Coaptite has also been used in the pediatric population for vesicoureteral reflux and has been found to be quite safe in multicenter prospective trials [44].
Silicone (Macroplastique)
Macroplastique, a hydrogel-suspended cross-linked polydimethyl-siloxane elastomer, has been approved by the FDA as a urethral bulking agent since 2006 for the treatment of SUI secondary to intrinsic sphincter deficiency [37]. This bulking agent is composed of relatively large silicone particles measuring 100 μm to 450 μm in diameter (mean diameter approximately 180 μm) suspended in a non-silicone carrier gel that is excreted unchanged in the urine [16, 25, 56]. The silicone particles quickly become encapsulated in fibrin with minimal inflammation [16, 26]. Studies of this material in rat and canine models show that Macroplastique is not readily phagocytized by macrophages, and fibroblasts do not readily adhere to Macroplastique [56].
In a multicenter, randomized, controlled trial comparing transurethral Macroplastique to bovine collagen for treatment of intrinsic sphincter deficiency, a greater proportion 61.5 % (75/122) of the patients receiving Macroplastique had an improvement of at least one Stamey Grade compared with 48 % (60/125) receiving bovine collagen (p < .001) at 12-month follow-up [20]. Additionally, a greater proportion of the patients receiving Macroplastique were dry (Stamey grade 0) compared with those receiving bovine collagen (36.9 % versus 24.8 %, p < .05). The total number of subjects who underwent two treatments in the 12-month follow-up was the same for both groups (Macroplastique 52.5 % and bovine collagen 58.4 %, p = .35), and the treatment volumes were not significantly different between the Macroplastique and bovine collagen groups. Additionally, Incontinence Quality of Life scores (I-QOL) improved in a similar magnitude from baseline between the Macroplastique and bovine collagen groups. Overall, adverse effects were similar between the two treatment groups (59 % and 54.5 %) with urinary tract infection (23.8 % versus 24.8 %), dysuria (9 % versus 8 %), urgency (9 % versus 7.2 %), frequency (8.2 % versus 9.6 %), and urinary retention (6.6 % versus 3.2 %) being the most frequently reported in the Macroplastique and bovine collagen groups, respectively. Only one serious adverse event (pyelonephritis) was reported in the bovine collagen group.
Ghoniem et al. reported 2-year follow-up data on the above cohort of patients who had received Macroplastique [21]. Of the 67 patients included in this 2-year follow-up, 67 % were dry (Stamey grade 0). Of the 38 patients who were dry at 12 months, 33 (87 %) of these remained dry at 2 years. Overall and subscale I-QOL scores at 24 months were also improved significantly over baseline for the 2-year follow-up group (p < .001). The conclusions from this ancillary study are limited, as they only followed the Macroplastique patients for the additional 1 year, and only reported on 55 % (67/122) of the original Macroplastique cohort.
Bulking Procedure
Patient Characteristics, Indications, and Contraindications
In general, most patients are thought to be ideal candidates for urethral bulking if they have SUI associated with intrinsic sphincter deficiency, defined as a valsalva leak point pressure (VLPP) <60 cm H2O or maximum urethral closure pressure <20–25 cm H2O, with at least 150 mL bladder fill, and an immobile urethra. There is evidence, however, that bulking agents are effective in cases of SUI associated with higher VLPP or in cases where SUI occurs with bladder neck hypermobility [6, 7, 58]. Currently, Medicare requires a LPP ≤ 100 cm H2O for procedural coverage. Other patients for whom bulking agents are considered are those who are medically compromised and cannot undergo a midurethral sling or have failed a prior surgery for stress incontinence [18, 32, 35]. In general, patients who are not considered to be optimal candidates for urethral bulking are those with urinary tract infections, urethral diverticula, poor urethral blood supply, high baseline post void residual urine volumes, severe detrusor overactivity, and reduced bladder capacity (<250 mL).
Necessary Equipment
The exact equipment required for a bulking procedure varies according to which agent you are utilizing for the procedure. In general, one needs a sterile prep solution for the vagina and peri-urethral tissues, 2 % lidocaine hydrochloride jelly (Uro-jet), 1 % lidocaine for peri-urethral blockade (if desired), sterile water, irrigation tubing with stopcock, camera, light cord, and cystoscopy tower. The type and size of endoscope utilized for peri-urethral bulking varies according to the bulking product.
Procedural Overview
The patient has a urinalysis upon arrival on the day of the procedure to assure there is no underlying urinary tract infection, and the procedure is cancelled if urinalysis suggests an existing urinary tract infection. According to the 2013 American Urological Association (AUA) Guidelines for urologic surgery antimicrobial prophylaxis, a prophylactic antibiotic (either a fluoroquinolone or trimethoprim-sulfamethoxazole) is given (http://www.auanet.org/common/pdf/education/clinical-guidance/Antimicrobial-Prophylaxis-PocketTable.pdf) [2]. Alternative antimicrobials include an aminoglycoside with ampicillin, first or second generation cephalosporin, or amoxicillin/clavulanate.
After informed consent and procedural time out are performed, the patient is placed in the dorsal lithotomy position, and betadine is used to perform a sterile preparation of the peri-urethral region and perineum. Local anesthetic, typically one 2 % lidocaine Uro-jet, is administered to the urethral lumen. Onset of anesthesia is typically 3–5 min. Additionally, a peri-urethral blockade with injectable local anesthetic, such as 1 % lidocaine, can be preformed. If this is done, injection at 3–4 o’clock and 8–9 o’clock is recommended, with approximately 4 mL of lidocaine used on each side [12].
Using a 30° operative cystoscope (lens degree can vary), the bulking agent is injected into the submucosa at two or more sites at the same level of the proximal urethra. Bulking agents can be injected just distal to the bladder neck or at the midurethra, although there is little data to support either approach. The efficacy of midurethral and bladder neck placement of urethral bulking agents has been compared in a small (N = 30), randomized study utilizing transurethral collagen [33]. At 10 months postoperatively, patients’ satisfaction on a visual analog scale did not significantly differ, and the proportion with negative cough stress tests did not differ between the bladder neck and midurethral groups (60 % versus 66 %, respectively). Bulking agents can also be injected peri-urethrally through the perineum, while viewing the bulking effect simultaneously with a cystoscope. The procedure for peri-urethral injections is discussed in the Procedural section for Durasphere EXP below. Most clinicians, however, seem to feel more comfortable with transurethral injections.
After the clinician has delivered what is perceived to be an adequate volume of the bulking material so that the urethral lumen coapts (Figs. 13.1 and 13.2), the patient is asked to cough. If transurethral urine loss is witnessed, additional bulking material may be administered. Once the bulking procedure is finished, the patient is asked to void. If she cannot void spontaneously, intermittent self-catheterization with a small diameter (8–12 French) catheter is taught. Clinicians may also prefer to teach intermittent self-catheterization prior to the procedure. Patients are typically given home-going instructions and precautions by the nursing staff and treating physician. Follow-up is typically arranged at 1–3 months, and patients are encouraged to call with any concerns.
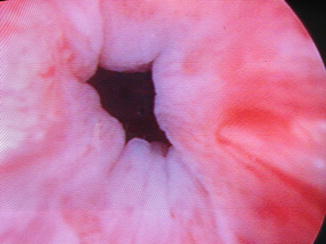
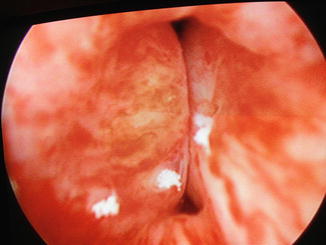

Fig. 13.1
Urethra prior to urethral bulking (Image courtesy of Drs. Mark Walters and Cecile Unger)

Fig. 13.2
Urethral coaptation after urethral bulking (Image courtesy of Drs. Mark Walters and Cecile Unger)
Tips and Tricks for Specific Bulking Agents
Each urethral bulking agent and injection system has its own unique features and challenges. Please refer to Table 13.2 for a summary of currently available bulking agents and their corresponding supplies. The following text summarizes instructions pertinent to each specific agent.
Table 13.2
Currently FDA-approved agents for peri-urethral bulking
Bulking agent (FDA approval) | Trade name (manufacturer) | Gauge needle | Syringe for agent | Injection locations (typical total volume) |
|---|---|---|---|---|
Glutaraldehyde cross-linked bovine collagen (1993) | Contigen™ (Bard, Inc.)a | 22–23 g | 2.5 mL | (2.5–5 mL) |
Pyrolytic carbon coated graphite beads (carbon) (1999) | Durasphere EXP™ (Coloplast, Inc.) | Transurethral 18/20 g 15 in. | Transurethral 1.0 mL | Between 4 o’clock 8 o’clock |
Peri-urethral 18/20 g 1.5 in. | Peri-urethral 3.0 mL | At 3 and 9 o’clock | ||
(2–4 mL) | ||||
Calcium hydroxylapatite (CaHA) (2005) | Coaptite™ (Boston Scientific) | 21 gb | 1.0 mL | 4 o’clock |
8 o’clock | ||||
(2–4 mL) | ||||
Polydimethylsiloxane particles (silicone) (2006) | Macroplastique™ (Uroplasty) | 18/20 g | 2.5 mL | 6 o’clock |
2 o’clockc | ||||
10 o’clockc | ||||
(5.5 mL) |
Durasphere (Durasphere EXP® Office Procedure Guide) [15]
Transurethral Injection
Preparation
Hold needle by its wings, and align the arrow on the 1 mL syringe tip with the dark bar located on the needle hub
Turn the syringe to connect the needle to the hub
Move the hand on the syringe back to finish tightening the syringe to the needle with a 360° rotation, until the arrow is aligned with the dark bar located on the needle
Prime the needle
Needle Placement
Chose a position between 4 and 8 o’clock
At the level of the midurethra, position the needle bevel toward the urethral lumen
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


