Reported practices
Associated potentially serious consequences
• Unmonitored PSA during magnetic resonance imaging (MRI)
• Not discovering in time potentially dangerous side effects (respiratory depression, hypoxia or bradycardia)
• No formal monitoring, observation, or assessment during recovery following the procedure
• Not discovering in time potentially dangerous late side effects. Immediately after the procedure, procedural stress falls away while sedative effect is still present. This may cause suddenly and unexpectedly deep sedation and loss of control on vital functions
• Deeply sedated patients not accompanied by a professional competent in airway management
• Not discovering and/or managing in time potentially dangerous side effects
• Waking up or moving during MRI
• Incomplete and/or low-quality results, limiting diagnostic accuracy
• Being called for additional intravenous sedation in a child sedated with chloral hydrate for MRI
• Procedural delay
• Risk of oversedation and undesirable deep sedation, associated with loss of control on vital functions
• Combination of preprocedural feeding, swaddling, and sedative drugs in infants undergoing MRI
• Risk of vomiting and aspiration
• Absence of age-specific resuscitation tools and drugs
• Not being able to start rescue interventions instantly
• Forced restraint during endoscopy procedures or oncology procedures (e.g., bone marrow puncture) because of ineffective PSA (mostly midazolam only)
• Extreme patient discomfort
• Preprocedural anxiety for new procedure
• Ineffective procedure
• Incomplete endoscopy procedures because of ineffective PSA
• Ineffective procedure leading to incomplete diagnosis and/or need for repeated endoscopy
• Nonapplication of topical anesthesia (e.g., EMLA®) in nonurgent vascular access
• Patient discomfort
• Preprocedural anxiety for new procedure
• Ineffective procedure
Monitoring Practices in Europe Are Inconsistent
Capnography
Capnography is becoming more frequently used for PSA in specialist centers [45]. The United Kingdom National Audit Project 43 was a survey of individual reports of major complications of airway management related to anesthesia, intensive care, and emergency medicine. The project found many examples of airway complications that could have been avoided or better managed if capnography had been used. This finding, albeit a professional opinion (rather than clear evidence of benefit), supports the widespread use of capnography in the management of intubated patients. It is logical to extend the use of capnography to monitor all patients who are either unconscious or at risk of becoming unconscious. A study from Turkey promotes its value in maintaining safety [46], and also elsewhere in Europe capnography is increasingly considered as an essential tool during PSA [47]. The Dutch guidelines on pediatric PSA (2012) recommend that capnography should be considered whenever PSA is performed with a (possibility of) moderate or deep sedation but that it is mandatory for any PSA during which continuous visual and auditory observation is impossible or unreliable (e.g., during an MRI investigation or during radiotherapy) [14]. Limited financial resources, however, have prevented widespread adoption of capnography.
Processed EEG
A recent advisory from the United Kingdom National Institute for Clinical Excellence (NICE) has stated that an EEG monitor should be considered for monitoring patients under anesthesia [48]. EEG monitoring is more common for total intravenous anesthesia because there are no monitors to follow the blood concentration of intravenous sedatives/anesthetics. Expired propofol measurement is possible, but not accurate enough to be a reliable tool [49, 50]. Although blood propofol assay machines are becoming available, they are not practical for standard short propofol PSA [51]. Bispectral index (BIS) monitoring and other electroencephalogram (EEG) monitors remain uncommon in European operating rooms.
Recommendations, Policy Statements, and Guidelines in Europe
Anesthesiologists throughout the world have been concerned about sedation by the untrained and have published guidelines to prevent patient harm. (Refer to Chap. 2.) Excluding dentistry, United Kingdom guidelines for doctors focused first on the radiology setting [52]. In 2001 the Academy of Medical Colleges responded to reports of unacceptable mortality in adult patients having esophagogastroscopy [53]. They stated clearly that “organizations should ensure that staff receives sedation training.” To date, there are no universal guidelines to encompass all of Europe.
The Scottish Intercollegiate Guidelines Network (SIGN) [54] gathered a body of opinion from across many specialties and developed a clinical guideline that has been quoted and used widely. The SIGN guideline was limited to moderate sedation. In 2010 NICE issued a comprehensive guideline specifically for children and young people, and it incorporated guidance for all forms of sedation including deep sedation [55]. In Italy, a review and guideline was produced for pediatric neuroradiology [56]. A European guideline for PSA in adults has been published [57]. Evidence-based national guidelines are now available in the United Kingdom, the Netherlands, Germany, and France.
In a survey we recently performed among about 100 participants of symposia on procedural sedation during two recent European conferences (PREM, Ghent May 2013; ISSP, Stockholm June 2013), the vast majority was not aware of any national or European guidelines. The lack of an appropriate, well-tailored program for guideline awareness and implementation is likely to be an important factor in this ignorance. The European Society of Anesthesiology (ESA), the European Society for Pediatric Anesthesiology (ESPA), the Association of Paediatric Anaesthetists (APA), the Royal College of Paediatrics and Child Health, the European Academy of Pediatric Societies (EAPS), the European Pediatric Association (EPA), and the European Society for Pediatric Research (ESPR) are not involved in setting up, training, or implementing appropriate training for PSA by non-anesthesiologists.
We believe that had any of the available guidelines been applied, the aforementioned disasters would not have happened [58]. Although these guidelines may have already prevented many catastrophes, in the authors’ opinion they would benefit from endorsement and dissemination by the national and European specialty organizations.
Ethical and Legal Aspects of Sedation Care in Europe
Ethical and legal considerations become increasingly important in European pediatric health care. In children undergoing a medical procedure, professionals must weigh the need to perform that procedure against the child’s wishes to be left untouched. Most importantly, if the knowledge and technology to perform sedation/analgesia for this procedure easily and painlessly exists, one cannot justify merely restraining a terrified child for a painful procedure because of the cost or extra efforts involved.
Current European legislations usually hold that a young child (defined as <12 years in Dutch legislation; defined as below the developmental age of reasonable comprehension in most other European countries) is not autonomous—that is, he/she is not at liberty to refuse needed treatment, as long as informed consent is obtained from parents or caregivers. This reasoning is probably unhelpful and may be a misreading of the law. It has been postulated that only if society ultimately considers physical restraint to accomplish a medical procedure a violation of a child’s civil liberty—which is, for example, the case in Scotland—we will all become more committed to alternative solutions such as PSA [59]. Medical professionals should help parents and children understand the nature of a given procedure and the possible options for altering perception of that procedure—be they emotional support, hypnosis, distraction techniques, anxiolytic/analgesic medications, or general anesthesia. Perhaps most importantly, the consideration of these ethical principles requires providers to reconsider alternative plans for sedation of each child: Physical immobilization or restraint cannot be a surrogate to sedation. Fear of potentially unsafe deep sedation is important but must be counterbalanced with the risk of unwanted emotional and psychological injury. Horrific accounts of painful procedures without effective PSA have been linked to post-traumatic stress disorder [6, 59–61].
Recent jurisprudence in the Netherlands shows that a care provider who does not allow sufficient time and effort to adopt a suitable approach to a resisting child may have to face negative consequences: Courts may rule that a defensive (panic) response from children resulting in injuries to the care provider is not unlawful. In these cases, any claim for damages against the parent(s) would fail [62]. Seen from the child’s perspective, it could furthermore be argued that the child has a right to oppose a medical treatment, at least within certain specific boundaries.
Alternatively, the ethical principle “first do no harm” and the basic right for optimal care require the PSA practice to be optimally safe at all occasions. The potential toxicity of PSA drugs needs to be excluded. To this end, the recent concerns on the possible neurotoxicity of anesthetics on the developing brain may be relevant [63]. Although clinical relevance has not been substantiated, results to date indicate that exposure of animals to general anesthesia during active synaptogenesis is most detrimental [64]. Given the recent trend to administer ketamine and propofol for PSA, these observations may be relevant. Currently it is not known whether the experimental findings in animals can be simply extrapolated to human beings in general and to PSA in children in particular. Furthermore, the eventual (neuro)toxicity of non-anesthetics such as barbiturates, benzodiazepines, and chloral hydrate has never been subject of systematic research. The potential toxicity of potent PSA drugs must be counterbalanced with the potential biological and psychological consequences of ineffective sedation and repeatedly painful or distressing experiences during childhood [65]. Additional research is needed and in progress in order to clarify this dilemma.
Definitions Particular to Europe
Conscious sedation was an accepted endpoint or landmark in the continuum of conscious level. Conscious, meaning “able to respond to the spoken word,” has been replaced by the term moderate sedation in the current literature because it does not assume consciousness but rather that the patient is easily roused—usually by communication but also by other similar appropriate light stimulus [66]. Nevertheless, conscious sedation remains a common term in Europe [52, 67]. In the United Kingdom, dentists prefer the term conscious sedation, referring to a level of sedation at which the patient responds easily to commands.
The term deep sedation is not approved [52] in some European professional groups, because it is possibly indistinguishable from anesthesia. This has led to the recommendation that both deep sedation and anesthesia should be managed with the same standard of care with respect to monitoring, equipment, facilities—and trained personnel. The definition therefore is more a description of the intended level of consciousness rather than a threshold identifying resources or risk. In a similar desire, two other descriptions of deep sedation/anesthesia have been used: Light anesthesia [68] and minimal anesthesia [69] are terms that describe a patient who is arousable with any appreciable stimulation. Techniques involving minimal anesthesia with propofol or sevoflurane have been used [70] for painless imaging.
Dissociative sedation is not a term in common use, but it is understood. Ketamine sedation or anesthesia is preferred generally.
Relative analgesia (RA) is a term intended to describe the analgesia and mild euphoria and calming properties of 30 % nitrous oxide. Dentists have become expert in its use [71].
The more relevant question is whether these definitions are useful. Although they may help identify sedation depths with drugs that are titratable, what is their value for non-titratable drugs? Motas et al. showed that common drugs (e.g., chloral hydrate, midazolam, pentobarbital) in average doses cause wide variations in depth of sedation [72]. The goal of achieving conscious or deep sedation was unable to be achieved in a significant number of children. With these findings in mind, the Dutch working group on procedural sedation decided to recommend the same safety precautions for all levels beyond light sedation.
Training and Credentialing Is Inconsistent in Europe
With the exception of dental sedation, there are no European training programs or specific qualifications for administering sedation. An Italian multicenter research group has reported the successful outcome of a strict training program for non-anesthesiologists who deliver propofol sedation to children [33, 39]. In the Netherlands, a national multidisciplinary training program (including the involvement of pediatric anesthesiologists) is currently being set up as to implement the PSA guidelines. In a first phase, participants will learn to perform light sedation (transmucosal midazolam, 50 % nitrous oxide inhalation), topical anesthesia, and hypnosis-like techniques. They will then progress to organize or manage procedures that require deep sedation (e.g., by setting up centralization or referral to anesthetists). The second phase is intended to give sedation providers sufficient exposure to become fully trained in deep sedation.
In the United Kingdom, an independent expert group has made recommendations on the training for pediatric dental sedation [73]. A challenge, however, is how to provide trainees with sufficient exposure to different, rarely used sedation techniques.
It is difficult to design a universal training curriculum for the many different types of sedation, some of which will not be relevant for all specialists. Strategies for credentialing have been clearly identified by Krauss and Green [74]. The authors of this chapter favor the option of creating a safe and effective sedation service that is controlled by the institution under direction from national and professional guidelines. Such a system will develop efficient training programs that may evolve into national training curricula.
All sedationists should have skills in airway management and resuscitation. Access to live patients is a limiting factor and the development of life-like manikins is a potential solution. (Refer to Chap. 35.) European resuscitation courses are widespread but do not aim to teach the monitoring and proactive airway skills that are critical for sedation providers. These skills should be an integral component of specialty-specific sedation training courses.
Implementation of Practice Standards in Europe
European standards of practice are mainly enforced by professionals themselves, and, unlike the United States, there are no financial penalties imposed by insurance companies. In the United Kingdom, clinical governance is a term applied in the National Health Service (NHS) to force individuals to bear responsibility and accountability for their actions. This governance has helped to improve quality and safety. In the United Kingdom and in the Netherlands, compulsory annual appraisal, and revalidation every 5 years, should motivate doctors and dentists to maintain their practice, skills, and knowledge. Failure to revalidate removes their license to practice.
Guidelines are designed to improve professional performance, health-care process, outcomes, and costs. However, the designing, publishing, and dissemination of guidelines do not necessarily imply the intended positive change in daily practice [75]. Guideline recommendations can be directed at a heterogeneous population of professionals with different backgrounds, experience, knowledge, skills, opinions, and motivational beliefs (i.e., positive and negative perceptions, evaluations, and expectations). This bewildering heterogeneity of factors must be reconciled, as illustrated in a recent study [76]. Since all these factors separately may act as both facilitators and barriers for guideline implementation, a thorough assessment of their interactive effects is critical in the design and implementation strategy. In the Netherlands, and elsewhere, the implementation of guidelines on PSA has been encouraged by raising public awareness through media and charities.
Financial Aspects of Sedation Delivery in Europe
How willing are society, health-care authorities, and insurance companies to invest in improving PSA? Given the current global financial crisis, it will be essential to demonstrate that implementing a guideline saves money. Probably, the “burden” of necessary investments to achieve more effective PSA services (e.g., training, new professionals, accessibility of propofol and nitrous oxide, appropriate monitoring and recovery, timely availability on a 24-h basis) can be quite easily calculated and will create immediately strong barriers for change. Calculating the economic aspects of the benefits will be much harder, balancing the costs of the improvements against the costs of unsafe practice and ineffectiveness.
A few studies on pediatric PSA have identified economical costs as an outcome measure. In the 1990s, Kain et al. compared propofol-based procedural sedation with intravenous thiopental/pentobarbital sedation for children undergoing MRI. A preliminary cost analysis was applied to the clinical data obtained and to a theoretical model of a pediatric MRI center. Cost analysis of the propofol-based services revealed an added drug cost ($1,600.76 per year for the propofol group) but a significant savings in post-sedation care unit (PACU) nursing time ($5,086.67 per year) [77]. Ekbom et al. published a randomized controlled study in children with difficulties in establishing venous access or anxious children in need for an IV access. The patients were randomized to conventional treatment—i.e., cutaneous application of Eutectic Mixture of Local Anesthetics (EMLA)—or nitrous oxide treatment. They concluded “the pre-treatment with nitrous oxide is a time effective and safe method to reduce pain, facilitate venous cannulation, and thereby reduce the number of costly cancellations of planned procedures” [78].
In 2013 the Dutch Association of Hospitals ordered a business-impact analysis and cost calculation on the implementation of the new Dutch PSA guideline. Their study showed that the implementation of deep sedation services for children undergoing major procedures (e.g., MRI, endoscopy, extensive wound care) in each of the 98 Dutch hospitals (on a total population of 16 million inhabitants) would not be financially effective. Centralization of these services to about 20 institutions would be financially more prudent. However, the same study demonstrated that setting up a 24-h sedation service to provide “light sedation” (e.g., application of nitrous oxide) for minor painful procedures would be cost-effective in all of the 98 Dutch hospitals.
The United Kingdom NICE guideline compared the costs of various sedation techniques and approaches [55]. (Refer to Chap. 2.) The most significant cost involved the salary for staff. This evaluation, however, was limited in its scope and applicability. For example, the comparison was based on a single procedure and did not consider the advantages of improved efficiency if, indeed, anesthesiologists were able to create “turnover” time.
Common European Sedation Practice for Selected Procedures
Painless Imaging
Both continents have tried to maximize the use of sedation for painless imaging. Nurse-led services for example were promoted as a practical alternative to anesthesia [79, 80]. Chloral hydrate [81] and Triclofos (Triclonam, Tricloryl, Nucloryl, Pedicloryl) [82] have been the mainstay for children under 15 kg and have very good safety and success records (safety depends upon the user more than the drug); 95 % of children fall asleep within 1 h and remain asleep for approximately 45 min. In older children, few drugs are as effective, leading most hospitals to abandon sedation in this group [83]. Pentobarbital was withdrawn in the United Kingdom in the 1960s due to its potential for abuse. Secobarbital has been used but causes paradoxical reactions (as in pentobarbital). Dexmedetomidine has recently become available but it is too early to know whether it will be widely used. It has been trialed extensively in Turkey [84, 85].
The unreliable nature of sedation has caused many, if not most, hospitals to develop anesthesia-led services [86] because there is a general belief that anesthesia is more efficient [87] and may be safer [88]. Certainly propofol [89] and sevoflurane [70] are standard drugs that are compatible with rapid recovery to street fitness. Propofol may need to be combined with other drugs to maintain immobility, and recently a combination of midazolam, nalbuphine, and low-dose propofol has been found to be reliable [90]. The centralization of nonurgent imaging in (mainly academic) pediatric anesthesia-led services has resulted, however, in progressively expanding waiting lists, making the need for non-anesthesiology involvement more prominent [91].
Interventional Radiology and Cardiology
Many intravenous lines can be inserted with a combination of local/topical anesthesia, moderate sedation, and behavioral techniques. There remain a large number of children who cannot remain immobile enough with deep sedation or anesthesia. Ketamine may be an alternative drugs but we believe that interventional radiology is more readily managed by an anesthesia service because of its flexibility and the ability to overcome almost any problem. For cardiology some countries have managed to maintain an effective sedation service using a range of techniques involving combinations of propofol [92], ketamine [93], and remifentanil [94], but our view is that the practice of controlled ventilation using tracheal intubation and standard anesthesia techniques is more reliable and creates optimal conditions for imaging and measurements [95, 96].
Gastroenterology
We believe that many hospitals in Europe use sedation for endoscopy with midazolam alone or a combination of benzodiazepines and opioids [97]. If there have been few problems, this is a credit to the judgment of gastroenterologists because the literature suggests that sedation is difficult especially for esophagoscopy [98]. Nevertheless, it is likely that most practitioners prefer anesthesia [99] and that propofol-based techniques are becoming more widespread. Propofol can cause sufficient sedation and suppression of gag reflex to allow insertion of an endoscope without the need for tracheal intubation or respiratory support [83]. Many anesthesiologists are confident that this is a safe approach [39, 83, 100, 101]. Colonoscopy needs much less propofol except when the ascending colon, the cecum, and the terminal ileum are entered (a small dose of opioid may be useful at these times). Not only is this technique a reliable and safe alternative to benzodiazepine-based sedation, but it radically increases the patient comfort as well as throughput [102]. In financial terms, there may be appreciable savings with efficiency.
Target-controlled infusion (TCI) of propofol has an application during endoscopy [103]. (Refer to Chap. 31.) The author (Sury), from personal experience, recommends a target of 6 μg/mL. It is important to appreciate that the effect site concentration is unknown and may take a few minutes to “catch up” with the blood concentration. A background infusion of remifentanil counters the discomfort of the procedure (usually less than 0.1 μg/kg/h). Nasal prongs delivering oxygen and monitoring breathing by capnography are essential to safety: Capnography is as important as pulse oximetry in this scenario. Respiratory depression and airway obstruction are uncommon complications, usually managed easily and not needing tracheal intubation.
Oncology
Many techniques are possible for children who need repeated painful oncology procedures. With practice, nitrous oxide combined with optimal topical/local anesthesia is potentially useful. In most countries we believe that intravenous anesthesia or deep sedation is preferred [104]. Without anesthesia services, ketamine is a reliable technique. The addition of a short-acting opioid to propofol is probably a common technique because it reduces the dose of propofol. In the Maastricht sedation unit, we recently found that children who had experienced both ketamine and propofol-based PSA for oncology procedures always select propofol-based PSA when they are given the choice. Unpleasant psychological experiences during recovery, double vision, the longer recovery time, and the relatively high incidence of nausea are the most important arguments for refusing ketamine. Propofol with remifentanil has the potential to provide the most rapid technique. It almost always causes apnea and assisted ventilation will be necessary; that it does cause apnea indicates that the child will remain immobile during the procedure [105]. The doses usually required to cause sleep and immobility for a 3-min-long painful procedure are propofol 2–3 mg/kg and remifentanil 1 mg/kg. TCI propofol may have an application for longer procedures.
Emergency Medical Care
There seems to be a gradual but steady progress by emergency physicians to develop their own standards and protocols, such that in Europe and in the United States hospitals support the use of ketamine [106], opioids, and propofol to manage children for minor procedures. There may be a trend for emergency departments (ED) becoming focused on quality and safety. However, PSA is currently not incorporated in European training programs. A recent European study showed that in most PEDs, PSA is practiced to the level of mild to moderate sedation. In about 20 % of the PEDs deep sedation is not provided by the staff, while 7.5 % of departments had no PSA available [107]. As a consequence, unnecessary or avoidable procedural pain and distress seems to be common [19, 108, 109].
Within most European countries, pediatric emergency care is not yet considered as a separate specialty and is performed by a mixed group of professionals from diverse disciplines. We hope that further professionalization and training will lead to the implementation of safe and effective PSA services in the ED. Some hospitals have made extra efforts to provide anesthesia services, usually at fixed times of the day, to meet maximum demand [110].
In the United Kingdom, a ketamine protocol has been produced by the College of Emergency Physicians.4 It is a clear and explicit guideline that seems to have provided a good safety record. It is generally appreciated that ketamine alone is a more effective reliable and safer technique than the combination of midazolam and fentanyl [111].
Dentistry
Dentists have been pioneers of sedation and many are expert. They know that during conscious sedation the patient should be rousable by verbal command and, in addition, they have observed that the mouth closes during deeper sedation.
Nitrous oxide relative analgesia (RA) has been popular because it is remarkably safe and surprisingly well tolerated by children [112]. In children who tolerate nitrous oxide, gas mixtures with less than 30 % nitrous oxide are almost always effective. More than this causes dysphoria, dizziness, and nausea [37]. Hypoxia is so unlikely that pulse oximetry and fasting are considered unnecessary (large meals beforehand are discouraged however) [113]. Nitrous oxide given in a 1:1 mix with oxygen has been used in many children for a variety of procedures [21]. Hypoxia was rare, as was any airway obstruction, and these problems only occurred when the patient had a cerebral disorder or was having another sedative drug [22].
Standard sedation for children is limited to nitrous oxide relative analgesia (RA) in most parts of Europe [114]. When nitrous oxide is insufficient to calm a patient, other drugs have been added. These may tip the patient into deep sedation, which is an obvious hazard, even though the risk may be small. In a study comparing RA with a combination of RA and 0.1–0.3 % sevoflurane, the dental treatment was completed in 52 % and 89 %, respectively. The same team, in another study, found that sevoflurane (0.3 %) added to nitrous oxide (40 %) and intravenous midazolam was effective in 93 % (249/267) of anxious children who would have been given general anesthesia otherwise [115]. All children remained rousable and none required airway management or oxygen—nevertheless, all children were fasted and monitored and these techniques were delivered by trained anesthesia personnel in a specialist dental clinic.
Other dentists have tried oral drugs. Oral and rectal benzodiazepines are commonplace in Sweden [116]. Midazolam is often useful to calm children [117] but treatment may have to be limited to minor restorations only [118]. In uncooperative toddlers (2–4 years old) an oral mixture of chloral hydrate, meperidine, and hydroxyzine was effective in only 72 %, and adverse conditions including vomiting, desaturation, prolonged sedation, and an apneic event occurred in 3 % of all sedations [119]. Nasal midazolam also has a place [120].
Intravenous midazolam alone is recommended in the United Kingdom for anxiolysis in children over 16 [114] and may be appropriate and effective in younger adolescents [121]. Propofol has been used alone as a sedation technique but lacks the analgesic component to enable insertion of local anesthesia [122]. Consequently, intravenous oral mixtures containing midazolam, alfentanil, ketamine, and propofol are being explored [123, 124]. A recent review of experience in 1,000 cases shows that these drugs can be combined safely [125]; loss of verbal contact occurred in approximately 0.05 % and nausea was a problem in 5 %. Whether this “alternative” technique can be called sedation is debatable if it is unknown whether it will cause accidental anesthesia. Some practitioners have become very experienced with combinations of drugs with ketamine [126, 127]. The danger of potent opioids causing apnea when the pain of dental treatment has subsided is a concern [42].
Conclusion
New and Future Developments
The NICE guideline—Sedation for diagnostic and therapeutic procedures in children and young people—has been developed in the United Kingdom and published by NICE in December 2010 [55, 130] incorporates evidence of safety and efficacy of selected sedation drugs, consensus statements about patient management, and cost-effectiveness considerations. Important deviations in these guidelines from those of the United States are the recognition of propofol and sevoflurane as potentially useful techniques for pediatric sedation [130]. The crucial statement is that “healthcare professionals trained in the delivery of anesthesia may administer sevoflurane, propofol, or a combination of opioids with ketamine” [130]. A treatment pathway and sedation algorithm is detailed in Fig. 21.1. (Refer to Chap. 2.)
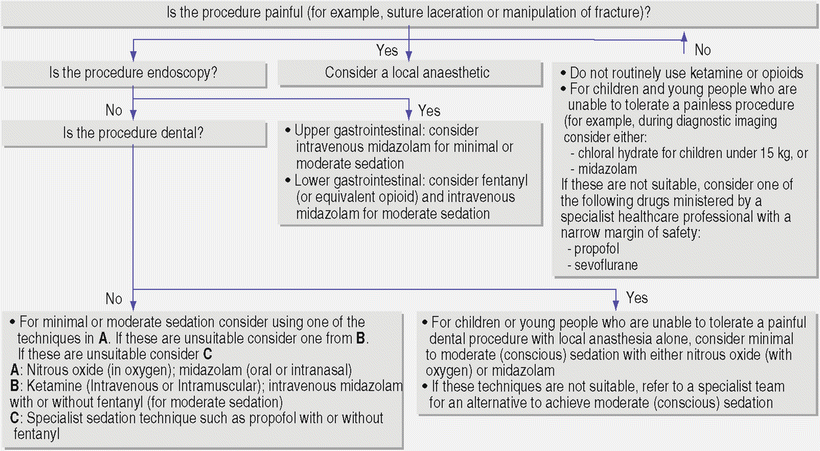

Fig. 21.1
Sedation algorithm and pathway (Reproduced from Sury M, Bullock I, Rabar S, Demott K. Sedation for diagnostic and therapeutic procedures in children and young people: summary of NICE guidelines. BMJ. 2010;341:c6819 with permission from BJM Publishing Group Ltd.)
In the Netherlands, the Dutch Institute for Healthcare Improvement (CBO) commissioned pediatric guidelines for PSA at locations outside the operating theater from the Netherlands Society of Anesthesiologists and the Dutch Society of Pediatrics [14]. (Refer to Chap. 2.) Recently published in 2012, the guidelines were meant to represent five important cornerstones, notably including the optimal use of local or topical anesthesia, non-pharmacological techniques, and the prohibition of forced securing and restraint [14] (Table 21.2). These guidelines were noteworthy because they distinguished deep sedation from dissociative sedation [14] (Table 21.3). However, for the sake of achieving consistent safety standards, only two sedation levels were retained in the final implementation plan: Regarding monitoring, fasting status, and professional competences, the same safety precautions apply for all levels beyond light sedation/anxiolysis. Basically the safety standards for any PSA regimen that (potentially) causes deep sedation should be similar.
Table 21.2
Cornerstones of a comprehensive policy toward optimal procedural comfort and the avoidance of forced immobilization (restraint) in children, Dutch Institute for Healthcare Improvement [14]
Strategy | Examples |
|---|---|
Preventive measures | Avoid superfluous procedures |
Only allow an experienced professional to carry out procedures | |
Agree on a maximum number of attempts at the procedure in advance | |
Early insertion of a central venous line under general anesthesia (e.g., during long-term treatment with intravenous antibiotics) | |
Optimal local and topical anesthesia | Allow sufficient time for topical anesthesia to become effective (e.g., at least 60 min for EMLA®) |
Apply topical anesthesia to the correct location | |
Implementation of new topical anesthetic techniques [14] | |
For infiltration with lidocaine: buffer lidocaine with bicarbonate and use the smallest possible needle to significantly reduce the pain upon infiltration [15] | |
Non-pharmacological techniques | Optimal positioning of the child [16] |
Presence of the parent(s) or guardian(s) | |
Preparation, game therapy | |
Distraction techniques and hypnosis | |
Ready availability of effective procedural sedation and/or analgesia (PSA) | Light sedation for “small” procedures (e.g., blends of nitrous oxide and oxygen) |
Deep, titratable sedation for very painful procedures (e.g., propofol) | |
Professionals trained in PSA | |
Rescue anesthesia | Availability of anesthesia if other techniques appear or turn out to be ineffective or unsafe |
Table 21.3
Definitions of different levels of sedation, Dutch Institute for Healthcare Improvement
1. Light sedation/anxiolysis | Two states that are difficult to tell apart, in which the anxiety and stress level of the patient have been lowered while the patient remains basically fully conscious. The patient responds adequately and consistently to verbal stimuli, and verbal communication therefore remains possible. This state is associated with few risks in patients without significant comorbidity. Although cognitive functions and coordination are reduced, ventilatory and cardiovascular functions remain unaffected. Light sedation/anxiolysis is typically a state of mind that occurs after one standard dose of midazolam (0.1 mg/kg intravenously or 0.2–0.5 mg/kg transmucosally) and with nitrous oxide sedation (inhalation concentration up to 50 %). Higher doses, other medicines, and combinations with other analgesics will virtually always lead to a deeper sedation level |
2. Moderate sedation | Pharmaceutically induced reduction in awareness, during which the patient still responds purposefully when spoken to, or to light tactile stimuli. In this stage, no interventions are needed to keep the airway open, airway reflexes are intact, and ventilation is adequate. If the response is not clearly adequate and purposeful but more of a withdrawal reflex, we speak of deep sedation |
3. Deep sedation | This is a pharmaceutically induced decline in awareness, during which the patient does not respond to being spoken to, but reacts purposefully to repeated or painful stimuli. Airway reflexes and ventilation may be reduced and it may be necessary to keep the airway open. The concept of “deep sedation” is a contested term because the distinction with anesthesia becomes less clear. A typical example is the deep sedation caused by propofol, during which it is possible, with the necessary expertise, to keep spontaneous respiration going and the airway open. The risk of reduced breathing is more or less a linear function of the dose and depth of sedation |
4. Dissociative sedation | Also called a trance-like cataleptic sedation, it is typically the result of sedation with ketamine. As far as the depth of sedation, analgesia, and response level is concerned, ketamine causes a state that primarily corresponds to anesthesia. However, contrary to anesthesia, the airway reflexes, respiration, and hemodynamics largely remain intact, even at comparatively high doses. It makes ketamine attractive for use in PSA, particularly for painful procedures |
5. General anesthesia | A pharmaceutically induced state of unconsciousness, in which the patient is unresponsive, even to painful stimuli. The ability to keep the airway open will often be reduced or absent, and ventilation will frequently be depressed, consequently requiring support. Cardiovascular functions may also be impaired. Can only be applied under the personal supervision of an anesthesiologist |
These Dutch guidelines discourage the delivery of sedation by non-anesthesiologists to American Society of Anesthesiologists (ASA) class III and IV patients. If performed, it should be after consultation with an anesthesiologist and delivered by a specially trained and credentialed practitioner. The fasting recommendation deviates from guidelines of other specialty societies in that light sedation does not need any special fasting. However, an emergency with a child who does not have an empty stomach is not an absolute contradiction for PSA [14] (Table 21.4).
Table 21.4
NPO fasting recommendations, Dutch Institute for Healthcare Improvement
1. Fasting is not needed for children undergoing light sedation |
2. A child must preferably have an empty stomach for any (elective) PSA with moderate or deep sedation, in accordance with the same guidelines that apply to interventions taking place under general anesthesia (2 h for clear liquids, 4 h for breastfeeding, and 6 h for other meals) |
3. A child in an acute condition without an empty stomach is in itself no absolute contraindication for PSA. This is important if postponing the procedure would pose health risks and/or discomfort. However, in that case the choking risks must always be carefully considered, taking into account the choice of sedative, the depth of sedation, and any protection of the airway. In practice, this amounts to the following recommendations: |
(a) With PSA in an acute situation (without an empty stomach), deep sedation must be avoided as much as possible, since the protective airway reflexes may be disturbed or there is a high risk of respiratory impairment |
(b) If a procedure requires a form of deep sedation, the patient must have an empty stomach |
(c) If a procedure requiring a form of deep sedation is urgently needed and an empty stomach can therefore not be guaranteed, deep sedation must be performed under the supervision of an anesthesiologist in order to ensure optimal protection of the airway |
4. Not having an empty stomach must be no reason or excuse for performing a procedure with an ineffective form of light or moderate sedation |
Propofol, in the Dutch guidelines, although preferably administered by an anesthesiologist, may be delivered by an experienced non-anesthesiologist to ASA class I and II patients. Patients of ASA class III status and higher can only receive propofol from an anesthesiologist [14] (Table 21.5). These guidelines are unique in that they have specific recommendations that are procedure based: Gastrointestinal procedures in particular should favor propofol, if necessary in combination with midazolam or an opioid [14] (Table 21.6).
Table 21.5




Propofol recommendations, Dutch Institute for Healthcare Improvement
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


