Fig. 11.1
Teaching tools
The physical and emotional care information that the family receives at the initial appointments can become very overwhelming. It’s the role of the ONN to assess absorption of information given at this initial appointment and continually update and reteach families about their child’s disease and treatment throughout the care continuum (Heiney and Wells 1995). Families are asked to bring their notebook for all admissions to enable teaching throughout treatment.
The bone tumor social worker is also notified of the initial appointment and meets with the family, completing an initial distress screening, and gives them her contact information.
After the patient leaves their initial oncology appointment, a referral is made to pediatric surgery for central line placement, and biopsy procedure is discussed with the surgeon by the primary oncologist. If indicated, a referral is made to the hematology/oncology procedure schedulers to add a bone marrow aspirate/biopsy/spinal tap to the central line surgery date. All efforts are made to combine all initial surgical procedures.
From the first appointment with the bone tumor team, family-centered care is initiated. The foundation of family-centered care is the understanding that the family is the true expert in the care of their child and the primary source of strength and support (Pearson 2009). It is through constant contact with the oncology team through the ONN that helps prepare and support the bone tumor patient and their family to be experts in their cancer care (Pearson 2009; Kelly and Porock 2005; Wilcox and Bruce 2010).
11.4 Treatment Initiation
11.4.1 Patient and Family Teaching
Prior to initiation of chemotherapy, the ONN meets with the family and patient and reviews all chemotherapy drugs, utilizing our institution’s teaching tools called Helping Hands, the APHON drug handouts, and the Children’s Oncology Group Family Teaching Notebook. This handbook can be located at: http://www.childrensoncologygroup.org/index.php/cog-family-handbook. All side effects are reviewed and a “when to call and who to call” tool with side effects is communicated to the family. The ONN reviews the discharge summary with the patient and family prior to discharge with each new chemotherapy drug, reinforcing side effects that may occur with each new drug. This tool also documents how to reach the ONN during office hours and the numbers to contact the oncology fellow on call after office hours and on the weekends. The ONN communicates with family members that this document may be good to keep on the refrigerator as a reference for anyone that may be responsible for the care of the patient. For the bone tumor patient receiving doxorubicin, the ONN discusses the EKG and echocardiogram and reviews with them when it will be repeated during treatment to assess for potential side effects. Knowing up front that these will be completed again tends to alleviate the anxiety that may occur if they arrive for their next chemotherapy admission that includes doxorubicin and they are getting an EKG and echocardiogram. Parents tend to think something is wrong, not that it is being completed to evaluate for side effects. Repeat audiograms for the patient receiving cisplatin are also explained. If applicable, treatment schemas are given to families to help them understand the timing of admissions and chemotherapy. Many patients and families utilize the schema to document chemotherapy treatments, as they mark off each one. This gives them something to look forward to as they can visualize an end to their child’s therapy.
On the patient’s second admission or visit for chemotherapy, the ONN reviews all lab values with the patient and family, including chemistries, complete blood count (CBC), and their urinalysis. During this time, she teaches the patient and family about their absolute neutrophil count (ANC), showing them how to calculate it as well as how it relates to the signs and symptoms of neutropenia. For those patients receiving highly emetic chemotherapy regimens, the ONN reviews closely the value of the BUN and creatinine that is completed and explains its ability to determine hydration status.
11.4.2 Patient Calendar
An ongoing calendar is created by the ONN and given monthly to help the patient and their family with grasping where they need to be. This calendar has become an effective teaching tool in helping patients and families understand their treatment schedule. Families frequently express that the calendar is used to guide them in making other family member appointments and schedules. Several families have stated that it is kept posted on the refrigerator at home so that everyone in the family knows the schedule. For the osteosarcoma patient receiving methotrexate, this calendar also instructs them when to hold or give their weekend Bactrim doses. The ONN also documents when counts are low and recovering on the calendar (see Fig. 11.2). Because it is an editable word document, the calendar allows for delays and can be updated accordingly. The calendar is often shared with other team members such as psychology and social work so they are aware of the patient’s upcoming appointments. The ONN also uses this calendar to teach delayed emesis medication teaching, when to start and stop their medications. If pegfilgrastim, filgrastim, or home care visits are being utilized, this is documented on the calendar as well.
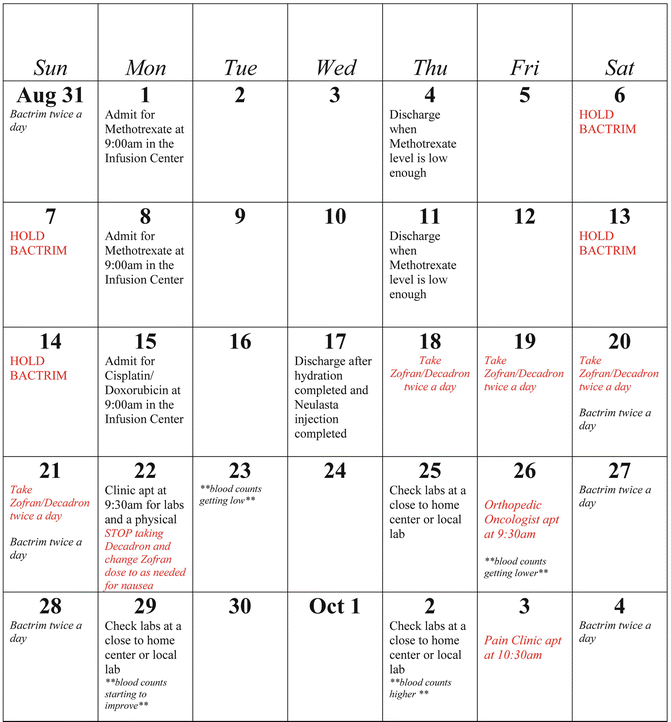

Fig. 11.2
Patient treatment calendar
It is essential that families understand the basics of their child’s disease; what to expect from their chemotherapy, biotherapy, radiation, and/or surgery; how to recognize adverse side effects; what is considered an emergency; and how to seek help (Lahl et al. 2008). They also need to understand all required tests and how to prepare for these tests, procedures, and blood work, in their role in delivering supportive care. Each family is unique and it is the responsibility of the ONN to effectively teach the patient and family by using verbal, written, and visual information that best meets their learning needs. The initial teaching is overwhelming, but the ONN has learned to go through teaching slowly, utilizing review and ongoing reinforcement, while allowing availability for questions. Most families are able to rise to the challenge (Lahl et al. 2008; Garcia 2014).
11.4.3 Home Medication Teaching
Prior to discharge, the ONN meets with the patient and family and reviews all home medications on their “Andrew’s medication list.” Each home medication name, dose, schedule, and purpose is listed on their medication list and reviewed (see Fig. 11.3). If the ONN realizes that the family is overwhelmed and anxious about the home medications, he/she will create a “MyMedSchedule” from the website: https://secure.medactionplan.com/mymedschedule/index.htm. This is a free application that allows a medication list with schedules that can be created, listing all medications hourly for the family. This application also shows a picture of the medication and can be created in English and Spanish. It also can be utilized as an app on a smartphone with medication due alerts and refill reminders. There are several other sites that can be utilized for medication teaching. Pill boxes can also be used for adherence to drug regimens. All prescriptions are verified with the bone tumor practitioner prior to discharge. The discharge instructions and follow-up are created by the ONN during each admission or visit to the clinic. The discharge instructions are reviewed with the patient and family. The instructions include side effects of each chemotherapy drugs they received and signs and symptoms of neutropenia, anemia, and thrombocytopenia, and who to call and when to call is clearly documented in the discharge plan. The ONN utilizes the electronic medical record to create “smart phrases” for each visit for chemotherapy. The smart phrases contain the discharge instructions and are shared with other team members to promote consistency in the information given to families.


Fig. 11.3
Sample home medication list
11.4.4 Managing Treatment Side Effects
11.4.4.1 Nausea and Vomiting
Nausea and vomiting are two of the most common side effects of chemotherapy and radiation treatment. Nausea and vomiting can be anticipatory, acute, or delayed (Lahl et al. 2008; Rheingans 2008) There is a variety of techniques to control nausea and vomiting. The most common treatment is the utilization of antiemetics. Treatment for nausea and vomiting occurs before, during, and after chemotherapy administration. Several categories of antiemetics are used in children and young adults with cancer (Lahl et al. 2008). There are also nonpharmacological techniques that can be used to aid in nausea and vomiting depending on their age. Utilizing an engaged psychosocial team that consists of child life (play) therapists, music therapists, massage therapists, and psychologists may help the patient using music, play, distraction, guided imagery or exercise to help them overcome their nausea and vomiting (Lahl et al. 2008).
Each patient’s antiemetic regimen is determined by the emetic potential of the chemotherapy or radiation treatment (see Table 11.1). Prevention of nausea and vomiting is always the goal for patients. Staying ahead of the nausea and vomiting produces better outcomes and a better quality of life for the patient. The ONN teaches the patient and family that treatment of nausea and vomiting is comparable to effective pain control. If you don’t stay prepared and ahead of it, it can spiral out of control and become difficult to gain relief. If nausea and vomiting is not well controlled on the first experience with chemotherapy, patients are more likely to experience anticipatory nausea and vomiting (Baggott and Association of Pediatric Oncology Nurses (U.S.) 2002).
Table 11.1
Emetogenic risk of therapies used for malignant bone tumors
High risk | Moderate risk | Low risk | Minimal risk |
|---|---|---|---|
Carboplatin Cisplatin Cyclophosphamide >1 g/m2 Dactinomycin Methotrexate >12 g/m2 | Cyclophosphamide <1 g/m2 Cyclophosphamide (oral) Daunorubicin Doxorubicin Etoposide Ifosfamide Irinotecan Methotrexate >250 mg to 12 g/m2 Temozolomide Vinorelbine | Docetaxel Doxorubicin (liposomal) Etoposide Gemcitabine Methotrexate >50 to 250 mg/m2 Nilotinib Paclitaxel Topotecan | Bevacizumab Dexrazoxane Sorafenib Sunitinib Temsirolimus Vincristine Vinorelbine |
Multiple agent risk | |||
Cyclophosphamide+ Doxorubicin Cyclophosphamide+ Etoposide Doxorubicin + ifosfamide Doxorubicin+ Methotrexate Etoposide + ifosfamide | |||
For delayed emesis, patients and families are instructed to utilize Zofran and Decadron twice a day for 4 days after receiving chemotherapy drugs that are highly or moderately emetic regardless if they feel nauseated or not. They are also instructed to utilize Phenergan or Ativan as a breakthrough medication. Each patient’s gastrointestinal responses need to be evaluated individually with each chemotherapy administration so their needs can be assessed prior to discharge and through post-discharge calls from the ONN. Guidelines established by the National Comprehensive Cancer Network (NCCN) – NCCN Clinical Practice Guidelines in Oncology for Antiemesis and the Pediatric Oncology Group of Ontario (POGO) and the POGO Guidelines for the Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients – are both resources utilized by the bone tumor team in their approach to the prevention of nausea and vomiting in children receiving antineoplastic agents.
Anticipatory nausea and vomiting (ANV) can occur any time after the first treatment, or sometimes even prior to the first treatment. The incidence and severity are influenced by the patient’s expectations of what is to come, as well as anxiety about nausea and vomiting. ANV most often begins within 2 hours before treatment and is most severe at the time of administration (Baggott and Association of Pediatric Oncology Nurses (U.S.) 2002). Managing ANV is crucial in controlling the patient’s nausea and vomiting throughout their treatment. The patient’s environment is important in determining the relative factors to their ANV. Psychogenic factors such as walking into the hospital, smelling particular odors, or the sight of a syringe can trigger nausea (Garcia 2014). Lorazepam administered at bedtime the night before chemotherapy treatment and again the following morning can diminish the distress and reduce the incidence and severity of anticipatory nausea and vomiting (Baggott and Association of Pediatric Oncology Nurses (U.S.) 2002).
Triaging nausea and vomiting from home can become frustrating for the patient and family. The ONN instructs the family and patient who are experiencing gastrointestinal toxicities to avoid foods that are high in sugar, processed foods, and fried or greasy foods. They are instructed to eat small meals that are easily digested (crackers, rice, toast, potatoes, or chicken) every 3–4 hours and to avoid the kitchen area or areas where there may be strong food odors. Pushing fluid intake in small quantities every 20–30 minutes, eating popsicles, slushies, or water are also techniques taught to the caregivers. Families are also instructed to utilize nonpharmocological interventions such as distraction, play, music therapy, and guided imagery while at home. The ONN teaches the patient and family to monitor for signs of dehydration such as few or no tears when crying, a dry or pasty mouth, cracked lips, sunken eyes, lack of energy, increased sleeping, dry skin, and concentrated, dark urine (Murphy 2011). If they have been unable to keep fluids down for 24 hours, are more lethargic, are urinating less, or are vomiting their medications, they are brought in to the infusion clinic or emergency department for evaluation.
11.4.4.2 Anorexia
Anorexia and cachexia are two of the most common nutritional side effects from cancer and its treatment. Factors contributing to anorexia may include nausea, vomiting, taste aversion from the chemotherapy or radiation treatments, anxiety, depression, mucositis, increased sensitivities to odors, and environmental changes (Lahl et al. 2008). Evaluation of the bone tumor patient’s weight and oral intake at each visit is crucial during treatment. The patient’s nutritional status is evaluated at the beginning of therapy by placing a consult to Clinical Nutrition Services. This nutritional screening is an ongoing process that must take into account patient weights, food intake, treatment that affects nutrition, functional status, physical examination, and lab results indicating nutritional biomarkers such as albumin and prealbumin levels. The initiation of Clinical Nutrition Services early in treatment aids in preventing malnutrition (Baggott and Association of Pediatric Oncology Nurses (U.S.) 2002). Nutrition management may include diet modifications, oral supplements, enteral feedings, parenteral nutrition, and/or medications to stimulate the appetite (Baggott and Association of Pediatric Oncology Nurses (U.S.) 2002).
11.4.4.3 Mucositis
Mucositis is caused by acute changes in the epithelium of the oral cavity resulting in the death or rapidly dividing epithelia cells (Eilers and Million 2007). Chemotherapeutic agents inhibit the growth and maturation of oral mucosal cells and disrupt the primary mucosal barrier in the mouth and throat. These changes can occur as early as 2–3 days after the administration of chemotherapy and peak in severity 7–10 days later, typically showing resolution within 2 weeks (Wohlschlaeger 2004). Chemotherapeutic agents that produce mucositis are listed in Table 11.2.
Table 11.2
Agents causing mucositis
Alkylating agents | Busulfan |
Cyclophosphamide | |
Ifosfamide | |
Melphalan | |
Procarbazine | |
Temozolomide | |
Thiotepa | |
Anthracyclines | Daunomycin |
Doxorubicin | |
Idarubicin | |
Mitoxantrone | |
Antimetabolites | Cytarabine |
5-Fluorouracil | |
Hydroxyurea | |
Mercaptopurine | |
Methotrexate | |
Thioguanine | |
Antineoplastic antibiotics | Bleomycin sulfate |
Dactinomycin | |
Nitrosoureas | Carmustine |
Lomustine | |
Plant alkaloids | Etoposide |
Paclitaxel | |
Vinblastine | |
Vincristine |
Oral mucositis is a frequent and potentially severe complication following chemotherapy and radiation. Not only is mucositis painful, but it can also cause impaired nutrition, infection, and treatment delays (Baggott and Association of Pediatric Oncology Nurses (U.S.) 2002; Eilers and Million 2007; Wohlschlaeger 2004). Mucositis has the potential to directly impact a patient’s quality of life. If it is severe, it may prevent adequate oral intake of food and fluids and the ability to take oral medications. It may sometimes lead to costly and unwanted hospitalization for parenteral nutrition, intravenous medications, and intravenous pain management (Wohlschlaeger 2004).
Due to the frequency of mucositis, teaching oral care to the patient and family prior to the beginning of their first chemotherapy treatment is a nursing priority. Patient education and participation are crucial since most patients are discharged after the completion of chemotherapy, and the monitoring and management of oral care and mucositis takes place at home (Eilers and Million 2007; Baggott and Association of Pediatric Oncology Nurses (U.S.) 2002). It is the ONN’s role to provide and supervise ongoing care and the triage of any complications after discharge. It is the goal of the ONN to manage the patient at home and avoid rehospitalization.
The first step of mouth care involves a thorough assessment by the medical team and a consult to dentistry prior to initiating treatment. Ongoing oral mucosa assessment with each treatment admission and prior to discharge allows the medical team to identify early oral lesions (Wohlschlaeger 2004). The second step is assessing their current, pretherapy oral care regimen and addressing any teaching needs or concerns. Next, the ONN instructs the family on a daily oral care regimen that includes brushing teeth four times a day, after meals and before bedtime with a soft toothbrush. Special instructions are given to patients who drink a lot of high-sugar sports drinks, requesting they brush after eating foods high in sugar. Mouthwashes can be used, but they should not contain alcohol. Parents and patients are taught to inspect their mouth daily for any signs of mucositis. They are instructed to look at the entire mouth: the lips, gums, tongue, side of the tongue, under the tongue, cheeks, roof of the mouth, and around the teeth. Avoiding spicy, bitter, salty, or acidic foods is another precaution for the patient (see Table 11.3). Keeping the mouth moist by eating popsicles, ice chips, slushies, smoothies, or cool fluids may also help with oral mucositis.
Table 11.3
Food considerations regarding mucositis
General guidelines | Recommended | Not recommended |
|---|---|---|
1. Small pieces of food | 1. Tepid or cool liquids | 1. Spicy, salty, bitter foods |
2. Use straw with liquids | 2. Puddings, Jell-O | 2. Dry foods (crackers, toast, chips) |
3. Nutritional supplements (e.g., boost, scandishake, ensure) | 3. Cottage cheese, soft cheeses | 3. Oranges, grapefruits, citrus fruits |
4. Topical analgesics prior to eating | 4. Bananas, peaches, applesauce | 4. Chewing gum, candy |
5. Mouthwash following meals | 5. Milkshakes, smoothies | |
6. Avoid hot foods | 6. Popsicles, ice cubes, Italian water ice |
Once the mucositis appears, pain is usually the first limiting symptom. The patient and family are instructed to continue with their current oral care regimen but may add sodium bicarbonate rinses (mixture of one fourth teaspoon salt, one fourth teaspoon baking soda, 4 oz of water), saline rinses or sterile water rinses every 1–2 hours, lip lubrication, and a mouthwash such as Philadelphia Mouthwash, containing a mixture of magnesium aluminum hydroxide, diphenhydramine, viscous lidocaine ,and water, that acts as a numbing agent to help with swallowing (Murphy 2011). They are instructed to take a pain reliever and then 20 minutes later use the Philadelphia Mouthwash to attempt at eating, drinking, or taking their oral medications throughout the day (Murphy 2011). Patients and families are instructed to call if they are unable to eat or drink secondary to the pain. While triaging the patient, the ONN assesses for hydration status; requesting volume of fluid intake, what does their urine look and how often have they urinated, is it less? Also, asking if the oral secretions are thick, are there a lot of oral secretions, any hoarseness or a “muffled” voice, any bleeding or nausea and vomiting, and lastly any airway stridor. If any signs of a compromised airway are identified within triage, the patient is asked to come in for evaluation, either to the outpatient clinic area or the emergency room. After evaluation by the medical team, the patient may begin antimicrobial treatment.
11.4.4.4 Fatigue
Defining fatigue has been a challenge, but ongoing pediatric fatigue research suggests that both children from 7 to 12 years of age and adolescents from age 13 to 18 years of age can effectively describe fatigue in terms of physical and mental symptoms (Baggott and Association of Pediatric Oncology Nurses (U.S.) 2002). Younger children described fatigue as a sad or mad face stating they feel tired or weak. Teens elaborated more, describing not only physical symptoms of sleepiness, nausea, dizziness, and a weakening of their body but also being mentally tired, feeling “not themselves,” and feeling sorry for themselves (Baggott and Association of Pediatric Oncology Nurses (U.S.) 2002). Contributing factors for younger children were associated with getting treatment, having pain, having low blood counts (anemia), changes in their sleep patterns, not being as physically active as they were pretreatment, and environmental factors while being in the hospital, such as interrupted sleep, or hearing noises and seeing lights on in the hallways. The adolescent patient identified the contributing factors relating to their fatigue as environmental: hearing noises, being in the hospital, going for treatment, nurses making noise, and young children making noise. They also related fatigue to personal/behavioral factors such as changing usual sleep patterns, changing sleep positions due to their disease or central line, doing too many things, being bored, having fears and anxieties, and being worried (Baggott and Association of Pediatric Oncology Nurses (U.S.) 2002).
Treatment strategies are varied, but regardless of the age of the patient, they all begin with a thorough assessment of the patient, including what they consider their causes of their fatigue (Lahl et al. 2008; Murphy 2011). The medical team can look for correctable causes such as anemia, pain, dehydration, or poor nutrition and work to alleviate or improve these conditions. A distinction is needed between fatigue and depression along with evaluating the patient’s pattern of activity during treatment (Baggott and Association of Pediatric Oncology Nurses (U.S.) 2002). The younger children identified that taking time out for short naps during the day, having visitors, and doing fun activities helped with their feelings of fatigue. Having an extensive psychosocial program that includes music therapy, art therapy, play therapy, and massage therapy can all contribute to helping with distraction and “fun activities” as mentioned by the younger children. The adolescent patient identified alleviating factors for their fatigue as going outdoors, having protected rest time, having fun with friends, taking naps, resting, and keeping busy. They also mentioned that treatments such as getting medications for sleep, having physical therapy, and getting a blood transfusion helped with fatigue symptoms (Baggott and Association of Pediatric Oncology Nurses (U.S.) 2002).
As healthcare professionals, we need to give the patients and families the permission to rest while they are in the hospital, letting them know that it’s okay to post a “nap time” or “rest time” on the door of their hospital room. For the many “technologically wired-in” patients that we care for, it’s also often about evaluating sleep hygiene. Making sure that all electronics are turned off at night so they can sleep well is a first step in evaluating sleep hygiene. Many times our patients cannot attend school full time, but allowing them to go for half days or even a few hours when their counts are good makes a huge difference in their attitude. Discussing their daily routines at home, cutting back on long naps during the day to manage better sleep patterns at night, and making a routine each day are other ways to combat fatigue. Creating nutritional plans and accessing the oncology dietician to help in creating adequate dietary needs with the goal of maximizing wellbeing can give energy to the patient experiencing fatigue. Maintaining adequate nutrition throughout the day is imperative for the patient experiencing fatigue. Instructing patients and families to eat small, high-calorie, high-protein snacks every 3–4 hours may help sustain the patient’s energy level (LaTour 2014). Exercise as a response to fatigue has shown significant strides in overcoming the symptoms of fatigue (Griffie and Godfroy 2014). Collaboration with the multidisciplinary team to keep the patient active during admits and while at home is another key to overcoming fatigue (Griffie and Godfroy 2014).
11.4.4.5 Pain
Pain is often the most common side effect for the bone tumor patient. If it is not well controlled, it can significantly impact the patient’s quality of life. For pain that is associated with tumor growth and sometimes even the occurrence of a pathological fracture, over-the-counter acetaminophen or ibuprofen become insufficient in controlling the pain. Medications that combine an analgesic and oral opioid are frequently the next step. The most effective intervention for relief of tumor pain is to initiate treatment as soon as possible (Pearson 2009) (see Table 11.4).
Table 11.4
Sources of pain in patients undergoing bone tumor therapy
Treatment related | Surgery related | Procedure related | |
|---|---|---|---|
Bone (primary or metastatic): bone marrow Central nervous system Neuropathic Somatic or visceral | Chemotherapy: Mucositis Peripheral neuropathy Aseptic bone necrosis Myalgias or arthralgias Extravasation injuries Bone pain Typhlitis Pancreatitis Constipation or bowel obstruction Radiation therapy: Mucositis Radiation dermatitis Myelopathy Radiation fibrosis Osteoradionecrosis Radiation-induced peripheral nerve tumors Radiation burns | Acute postoperative Post-thoracotomy syndrome: numbness or neuropathic pain that can occur after thoracic surgeries | Finger stick Venipuncture Intravenous cannulation Implanted central venous catheter Venous access device removal Suture removal Bone marrow aspiration Bone marrow biopsy Lumbar puncture LP-related headache or backache |
Initial pain assessment should include a self-report (location, character, time of worst and least pain, factors that improve and aggravate pain, and intensity utilizing a pain rating scale), parent input, observation of behavior, physical examination, and physiological measures/diagnostic results (Pearson 2009). Assessments in the future will often be compared to this initial baseline pain assessment. If pain resolves itself quickly after treatment begins, it is indicative of a positive response to therapy. If pain increases while receiving chemotherapy, either at the tumor site or other areas, it is troublesome in that it may indicate a lack of response to treatment. Frequently, imaging or pathology may confirm this concern. A sudden onset of acute pain without accompanying trauma may indicate a pathological fracture (Pearson 2009).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


