CHAPTER 17 Ovulation induction
Introduction
The aim of ovulation induction is to achieve development of a single follicle and subsequent ovulation in anovulatory infertile women. The ability to induce ovulation is possible in most women, with excellent conception rates. Anovulation accounts for 20–25% of the causes of infertility, and clinicians who manage infertile couples ought to have a sound understanding of the control of follicular development in a normal cycle (see Chapter 15), causes of anovulation (see Chapter 16), appropriate investigations and different treatment options, including their indications and risks. This chapter will briefly review the physiology of ovulation, including the basic principles of ovulation induction, and describe treatment strategies based on the underlying cause of the anovulation.
Principles of Ovulation
Follicular development: recruitment, selection and dominance
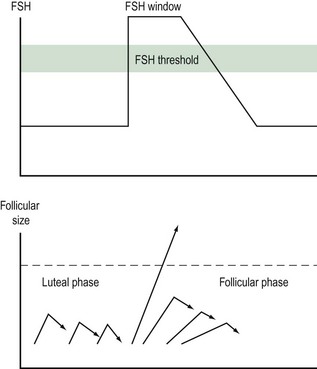
Follicular growth starts with the regression of the corpus luteum at the end of the previous cycle. As the levels of steroid hormones and inhibin drop, their inhibitory effect on FSH is abolished and FSH levels rise. This increase starts approximately 2 days prior to menstruation. As the follicles grow, the production of oestradiol and inhibin increases and FSH levels subsequently fall. The dominant follicle will be selected from day 5–7 of the menstrual cycle. The selection of a single dominant follicle appears to be the result of two oestrogen actions: a positive influence of oestrogen on FSH action within the maturing follicle, and a negative feedback mechanism on FSH at the hypothalamic–pituitary level. This latter action leads to a decline in gonadotrophin levels and ultimately to atresia of the less-developed follicles. Local paracrine–autocrine factors are also involved in this process, such as tumour necrosis factor and anti-Müllerian hormone. The dominant follicle is not affected by this decline in FSH levels, as it is more sensitive to FSH. Provided that a critical exposure of FSH was initially sustained, the dominant follicle continues to grow. In turn, FSH induces the development of LH receptors on the granulosa cells. Thus, LH plays a crucial role in the late stages of follicular development (Figure 17.1).
Ovulation Disorders
Classification
Investigations
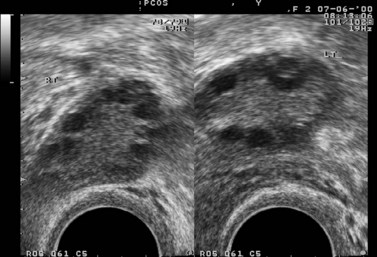
In the case of PCOS, the hormonal investigations should include serum androgens (testosterone, free testosterone, androstenedione and dehydroepiandrosterone sulphate), as they are often elevated, and baseline 17OH-progesterone. Depending on the results, adrenocorticotrophic hormone concentrations may be measured to exclude adrenal pathology. If PCOS is suspected, a transvaginal ultrasound should be performed to assess the ovaries and look for the characteristic peripheral follicles (‘necklace sign’). The criteria used for the ultrasonographic diagnosis of PCOS include the presence of more than 10 follicles with a diameter of 2–10 mm and increased density of the ovarian stroma (Adams et al 1986).
Diagnosis and Treatment
Based on the results of the above investigations, the causes of anovulation can be divided into four main categories (see Box 17.1).
Box 17.1 Classification of ovulation disorders
Hyperprolactinaemia
Causes of hyperprolactinaemia include:
Non-prolactin-secreting tumours are treated surgically in combination with radiotherapy.
Primary hypothyroidism causing hyperprolactinaemia is treated with thyroid replacement therapy.
Hypergonadotrophic hypogonadism
Hypogonadotrophic hypogonadism
Its causes can be either congenital or acquired. Kallman’s syndrome is one of the congenital causes. It consists of isolated gonadotrophin deficiency and anosmia. The syndrome is usually sporadic but it can also be inherited (see Chapter 16).
Acquired causes of hypogonadotrophic hypogonadism include:
If a central nervous system tumour is present, surgery is indicated.
Normogonadotrophic hypogonadism
This category includes women with anovulation with normal FSH, LH and prolactin levels. They may present with regular menses, oligomenorrhoea or even amenorrhoea. Predominantly, this group comprises women with PCOS. This is discussed in more detail in Chapter 18.
Other causes of normogonadotrophic hypogonadism and hyperandrogenism include:
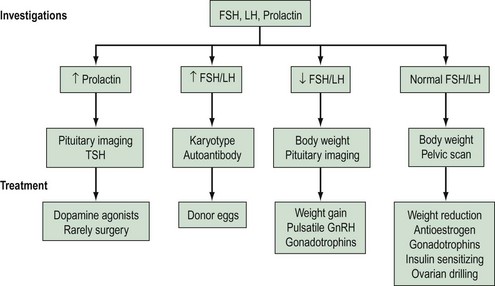
A summary of investigations, classifications and treatments is shown in Figure 17.3, and an overview of assessments and treatments is given in the guidelines of the National Institute for Health and Clinical Excellence (2004).
Antioestrogens
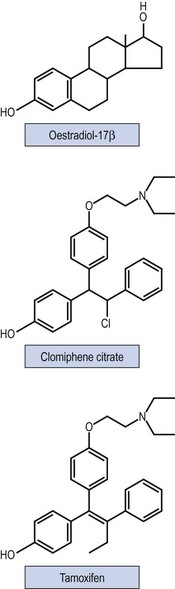
Non-steroidal selective oestrogen receptor modulators, including CC and tamoxifen, are used for ovulation induction. It is thought that they bind with oestrogen receptors in the hypothalamus, leading to a perceived drop in the endogenous oestrogen levels. As a consequence, GnRH and endogenous gonadotrophin secretion is increased, leading to induction of ovulation. Figure 17.4 shows the structure of the drugs.
Clomiphene citrate
Indications for treatment
CC has also been tried for the treatment of unexplained infertility. Nonetheless, when CC treatment was compared with placebo or no treatment, there was no evidence that CC was more effective in this subgroup of infertile women (Hughes et al 2000).
Treatment regimens and monitoring
If ovulation is not induced with the initial dose of 50 mg, the dose of CC can be increased in 50-mg increments in subsequent cycles up to a maximum dose of 250 mg/day. Approximately 70% of anovulatory women treated with CC respond to a dose of 100–150 mg/day (Gysler et al 1982). In practice, doses of 250 mg are rarely used. Women who do not ovulate while taking a dose of 150 mg are considered to be ‘clomiphene-resistant’ (Vandermolen et al 2001). The required CC dose is correlated to body weight, as there is a significant association between CC treatment failure and BMI greater than 27 kg/m2. Women who are overweight should be advised that a 5% reduction in weight may improve endocrine and ovarian function, increase spontaneous conception rates and improve the response to CC treatment. Lower doses of 25–50 mg/day should be considered for those women who are exceptionally sensitive to treatment with CC.
Monitoring of CC treatment is recommended. Ovulation is confirmed either with serum progesterone measurements or with the use of transvaginal ultrasonography. Serum progesterone concentrations are measured during the luteal phase. Ultrasound monitoring should be offered at least during the first treatment cycle to ensure that the woman is on the lowest necessary dose to achieve ovulation (National Institure for Health and Clinical Excellence 2004). Monitoring will minimize the risk of multiple pregnancy and ovarian hyperstimulation syndrome (OHSS), and is essential in patients in whom ovulation does not seem to have been induced.
Results
Studies with CC have shown an ovulation rate of 60–85% and a pregnancy rate of 30–40%. The discrepancy between ovulation and fecundity rates has not been explained, but may be due to other coexisting fertility factors, such as endometriosis or tubal damage. Some argue that it may be associated with possible antioestrogenic effects that CC exhibits on the endometrium and cervical mucus (Milson et al 2002).
A systematic review of 12 randomized controlled trials supported the effectiveness of CC therapy for anovulatory infertile women (Beck et al 2005). Clomiphene was found to be effective in increasing pregnancy rate compared with placebo [fixed odds ratio (OR) 5.8, 95% confidence interval (CI) 1.6–21.5; number needed to treat (NNT) 5.9, 95% CI 3.6–16.7].
Cumulative conception rates continue to rise after six cycles of treatment, reaching a plateau after 12 cycles. However, prolonged treatment with CC may be associated with an increased risk of ovarian malignancy. Therefore, after completion of 12 cycles of treatment, alternative therapies ought to be considered (National Institute for Health and Clinical Excellence 2004). Factors associated with increased risk of CC treatment failure include increased maternal age, raised BMI and history of severe oligomenorrhoea (Milson et al 2002).
Risks of treatment
With CC therapy, the risk of multiple gestation is approximately 2–13% (Venn and Lumley 1994). OHSS may occur in approximately 13% of cases, whereas severe OHSS is rare. Counselling of women undergoing ovulation induction with CC regarding the above risks is, therefore, essential.
The incidence of spontaneous miscarriage is 13–25% (Milson et al 2002). This figure is no different from the incidence of miscarriage in spontaneous conceptions. In addition to this, there is no evidence to indicate that CC treatment is associated with a higher incidence of congenital abnormalities.
Adjuvant treatment
A systematic review of 12 randomized controlled trials (Beck et al 2005) examined the concomitant administration of CC with other agents to infertile anovulatory women. The use of clomiphene in combination with tamoxifen did not provide any evidence of increased effect on pregnancy rate compared with clomiphene alone. The results were similar when CC plus ketoconazole was compared with CC alone, and CC plus bromocriptine was compared with CC alone.
Tamoxifen
Preliminary research has shown that tamoxifen has similar ovulation (44% with tamoxifen vs 45% with CC) and pregnancy rates (22% with tamoxifen vs 15% with CC) (Boostanfar 2001, Gerhard and Runnebaum 1979) compared with CC.
A meta-analysis, involving four trials, compared tamoxifen with CC in anovulatory women and showed that the two drugs were equally effective. Both drugs resulted in similar ovulation rates (OR 0.755, 95% CI 0.513–1.111). No benefit of tamoxifen over CC in achievement of pregnancy per cycle (OR 1.056, 95% CI 0.583–1.912) or per ovulatory cycle (OR 1.162, 95% CI 0.632–2.134) was demonstrated (Steiner et al 2005).
One randomized controlled trial showed that combination therapy of tamoxifen with CC did not improve pregnancy rates [8.6% with tamoxifen/CC vs 4.8% with CC alone; relative risk (RR) 1.80, 95% CI 0.20–16.21] (Suginami 1993).
Letrozole
Pharmacology and mechanism of action
Aromatase inhibitors were initially introduced for the treatment of breast cancer. Aromatase is a cytochrome P-450 haemoprotein that catalyses the rate-limiting step in the production of oestrogens using androgens as a substrate. Letrozole is a highly potent, selective and reversible aromatase inhibitor (Bayar et al 2006). Letrozole inhibits peripheral oestrogen production and, therefore, stimulates endogenous FSH secretion. Moreover, the accumulated androgens may increase follicular sensitivity to FSH (Mitwally and Casper 2004). Its high oral bioavailability and short half-life make it a suitable agent for ovulation induction. One potential advantage of letrozole over CC is its lack of direct antioestrogenic effects on the cervical mucus, the endometrium, uterine blood flow and embryo development (Barroso et al 2006).
Results
In a Cochrane review, Cantineau et al (2007) identified five trials comparing aromatase inhibitors with antioestrogens used as ovulation induction agents in ovarian stimulation protocols for intrauterine insemination (IUI) in subfertile women. No benefits of letrozole use were identified. Other studies involving women with anovulation or unexplained infertility have shown comparable pregnancy rates between letrozole and CC (Barroso et al 2006, Bayar et al 2006, Davar et al 2006, Jee et al 2006). One study reported higher pregnancy rates in PCOS patients treated with letrozole (Atay et al 2006). Results from larger randomized controlled trials are needed to determine the role of letrozole in ovulation induction.
Dopamine Agonists
Pharmacology and mechanism of action
Prolactin secretion from the anterior pituitary is mainly regulated by the inhibitory control of dopamine. Therefore, drugs that exhibit a dopaminomimetic activity reduce prolactin serum concentrations and restore gonadal function in hyperprolactinaemic women, with or without a pituitary adenoma. If a prolactinoma is present, dopamine agonists may also reduce tumour size (see Chapter 16).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree