Teri A. Longacre
Michael Friedlander
Of all the gynecologic cancers, ovarian malignancies represent the greatest clinical challenge because they have a high mortality. Epithelial cancers are the most common ovarian malignancy, and over two-thirds of patients have advanced disease at diagnosis. Ovarian cancer represents a major surgical challenge, and optimal therapy includes surgical debulking followed by platinum-based combination chemotherapy. It has the highest fatality-to-case ratio of all the gynecologic malignancies. There are nearly 22,000 new cases annually in the United States, and 15,460 women can be expected to succumb to their illness (1). Ovarian cancer is the seventh most common cancer in women in the United States, accounting for 3% of all malignancies and 6% of deaths from cancer in women and almost one-third of invasive malignancies of the female genital organs. Ovarian cancer is the fifth most common cause of death from malignancy in women. A woman’s risk at birth of having ovarian cancer at some point in her lifetime is 1% to 1.5% and that of dying from ovarian cancer is almost 0.5% (2).
Epithelial Ovarian Cancer
Approximately 90% of ovarian cancers are derived from the coelomic epithelium or mesothelium (2). The cells are a product of the primitive mesoderm, which can undergo metaplasia. A classification of the histologic types of epithelial tumors of the ovary is presented in Table 37.1. Neoplastic transformation can occur when the cells are genetically predisposed to oncogenesis or exposed to an oncogenic agent or both (3).
Table 37.1 Epithelial Ovarian Tumors
| Histologic Type | Cellular Type |
| I. Serous | Endosalpingeal |
| A. Benign | |
| B. Borderline | |
| C. Malignant | |
| II. Mucinous | Intestinal, Endocervical |
| A. Benign | |
| B. Borderline | |
| C. Malignant | |
| III. Endometrioid | Endometrial |
| A. Benign | |
| B. Borderline | |
| C. Malignant | |
| IV. Clear-cell | Müllerian |
| A. Benign | |
| B. Borderline | |
| C. Malignant | |
| V. Brenner | Transitional |
| A. Benign | |
| B. Borderline (proliferating) | |
| C. Malignant | |
| VI. Mixed epithelial | Mixed |
| A. Benign | |
| B. Borderline | |
| C. Malignant | |
| VII. Undifferentiated | May be anaplastic |
| VIII. Unclassified | |
From Seroy SF, Scully RE, Sobin LH. International histological classification of tumours no. 9. Histological typing of ovarian tumors. Geneva, Switzerland: World Health Organization, 1973, with permission. | |
Pathology
Invasive Cancer
Seventy-five percent to 80% of epithelial cancers are of the serous histologic type. Less common types are endometrioid (10%), clear cell (5%), mucinous (5%), transitional (Brenner), and undifferentiated carcinomas, with each of the last two types representing less than 1% of epithelial lesions (2). Each of the major tumor types is named on the basis of a histologic pattern that resembles epithelium in the lower genital tract (3). For example, serous tumors have an appearance similar to that of the glandular epithelial lining of the fallopian tube, endometrioid tumors resemble proliferative endometrium, and clear cell tumors resemble secretory or gestational endometrium. Mucinous tumors may contain cells that resemble endocervical glands, but more commonly these cells resemble the gastrointestinal epithelium. Transitional (Brenner) tumors are so named because of a resemblance to the epithelium in Walthard rests and bladder urothelium.
Although it was believed that epithelial ovarian cancers arise from either the surface epithelium of the ovary or from inclusion cysts within the ovary, there is growing evidence to suggest that many, if not most, high-grade serous carcinomas of the ovary arise from the fimbrial end of the fallopian tube rather than from the ovary (4,5). It is suggested that serous epithelial ovarian cancers be separated into two distinct groups—type I and type II serous tumors—as they differ considerably in the cell of origin, molecular pathogenesis, and their biological behavior (6). Type I tumors include serous borderline tumors and low-grade serous carcinoma; they are genetically stable and are characterized by mutations in KRAS and BRAF. Type II serous tumors are rapidly growing, highly aggressive neoplasms that lack well-defined precursor lesions; most are advanced stage at, or soon after, their inception and many appear to arise in the fimbrial end of the fallopian tube (7). The type II tumors are genetically unstable and harbor p53 mutations.
Borderline Tumors
An important group of tumors to distinguish is the tumor of low malignant potential, also called the borderline tumor. Borderline tumors are lesions that tend to remain confined to the ovary for long periods, occur predominantly in premenopausal women, and are associated with a very good prognosis (2,3,8–12). They are encountered most frequently in women between the ages of 30 and 50 years, whereas invasive carcinomas occur more often in women between the ages of 50 and 70 years (2).
Although uncommon, implants may occur with serous borderline tumors. Such implants are divided into noninvasive and invasive forms. The latter group has a higher likelihood of developing into progressive, proliferative disease in the peritoneal cavity, which can lead to intestinal obstruction and death (2,6).
Classification of Epithelial Ovarian Tumors
Serous Tumors
Serous tumors are so classified because they resemble tubal secretory cells. Psammoma bodies are frequently found in these neoplasms, and they are made up of concentric rings of calcification. Several hypotheses pertaining to the origin and development of psammoma bodies are proposed, including apoptosis of tumor cells and osteoinductive cytokines produced by macrophages (6). In the wall of the mesothelial invaginations, papillary ingrowths are common, representing the early stages of development of a papillary serous cystadenoma. There are many variations in the proliferation of these mesothelial inclusions. Several foci may be lined with flattened inactive epithelium; in adjacent cavities, papillary excrescences are present, often resulting from local irritants (2).
Borderline Serous Tumors
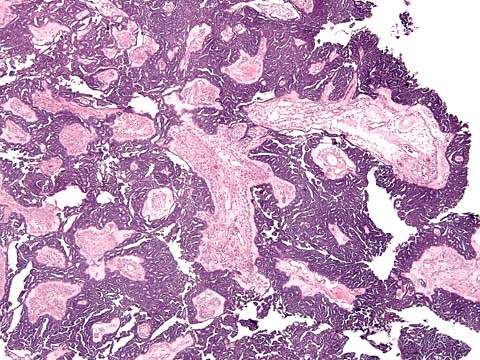
Approximately 10% of all ovarian serous tumors fall into the category of a tumor of low malignant potential or borderline tumor (Fig. 37.1), and 50% occur before the age of 40 years. The criteria for the diagnosis of serous borderline tumors are as follows (11):
Serous borderline tumors that are composed of an exuberant micropapillary architecture are designated as serous borderline tumors with micropapillary features (Fig. 37.2); these tumors are more frequently bilateral, exophytic, and high-stage than the usual serous borderline tumor.
Figure 37.1 Serous borderline tumor of the ovary. Complex papillary fronds with hierarchical branching are lined with pseudostratified columnar cells. The epithelium and the stroma are clearly separated by a basement membrane, indicating no stromal invasion.
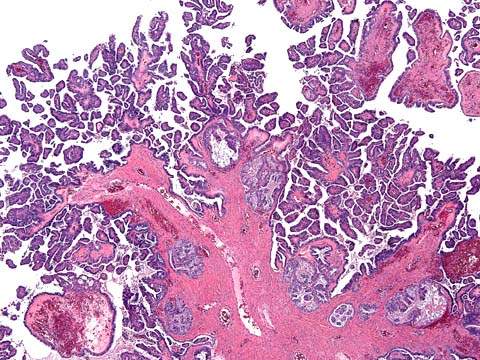
Figure 37.2 Serous borderline tumor with micropapillary features. The papillae have a non-hierarchical branching pattern and are lined by a monomorphous population of cells.
It should be emphasized that up to 40% of serous borderline tumors are associated with spread beyond the ovary, but high-stage disease does not necessarily warrant a diagnosis of carcinoma. The diagnosis of a serous borderline tumor versus serous carcinoma is based on the histologic features of the primary tumor (11). Up to 10% of women with ovarian serous borderline tumors and extraovarian implants may have invasive implants, and these can behave more aggressively (13). The 5-year overall survival for women with invasive implants is about 50% if stringent criteria are applied (10,13–15). Most implants are noninvasive (10,16). In the noninvasive implants, papillary proliferations of atypical cells involve the peritoneal surface and form smooth invaginations (10). In contrast, the invasive implants resemble well-differentiated serous carcinoma and are characterized by atypical cells forming irregular glands with sharp borders. Implants are usually confined to the abdominal cavity and may be seen in the pelvis, omentum, and adjacent tissues, including lymph nodes, but spread outside the abdominal cavity is rare. Death can occur as the result of intestinal obstruction (16–19).
Borderline serous tumors may harbor foci of stromal microinvasion (18). Most patients are young and International Federation of Gynecology and Obstetrics (FIGO) stage I. Stromal microinvasion is increased about ninefold in pregnant women with serous borderline tumors.
The presence of stromal microinvasion is associated with lymphovascular space invasion in the primary ovarian tumor (and likely represents a form of true stromal invasion), but it is not associated with an aggressive clinical course, and patients with this finding should be managed in the same way as patients without stromal microinvasion.
Serous Carcinomas
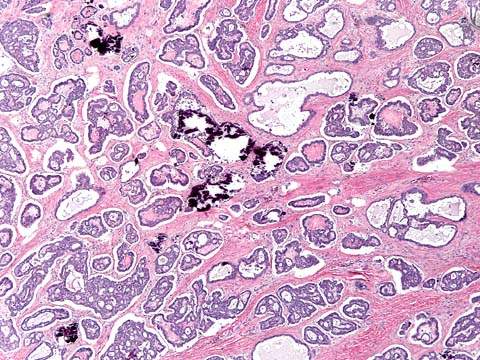
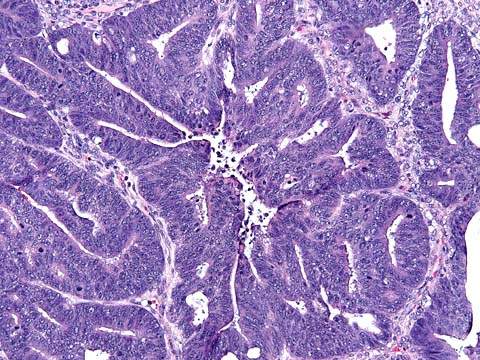
In malignant serous tumors, stromal invasion is present (2). The grade of tumor is important and needs to be documented. In low-grade serous adenocarcinomas, papillary and glandular structures predominate (Fig. 37.3); high-grade neoplasms are characterized by solid sheets of cells, nuclear pleomorphism, and high mitotic activity (Fig. 37.4). Laminated, calcified psammoma bodies are found in 80% of serous carcinomas. Serous psammocarcinoma is a rare variant of serous carcinoma characterized by massive psammoma body formation and low-grade cytological features. At least 75% of the epithelial nests are associated with psammoma body formation. Patients with serous psammocarcinoma have a protracted clinical course and a relatively favorable prognosis; their clinical course more closely resembles that of high-stage, progressive serous borderline tumor than serous carcinoma.
Figure 37.3 Low-grade serous adenocarcinoma of the ovary. Clusters and papillae of malignant cells are in direct contact with fibrous stroma indicative of stromal invasion.
Mucinous Tumors
These cystic ovarian tumors have loculi lined with mucin-secreting epithelium. The lining epithelial cells contain intracytoplasmic mucin and resemble those of endocervix, gastric pylorus, or intestine. They represent about 8% to 10% of epithelial ovarian tumors. They may reach enormous size, filling the entire abdominal cavity (2).
Borderline Mucinous Tumors
The mucinous tumor of low malignant potential is often difficult to diagnose. Although it is common to find a rather uniform pattern from section to section in the serous borderline tumor, this is not true in the mucinous tumors. Well-differentiated mucinous epithelium may be seen immediately adjacent to a poorly differentiated focus. It is important to take multiple sections from many areas in the mucinous tumor to identify the most malignant alteration (2).
Mucinous Carcinomas
Bilateral tumors occur in 8% to 10% of cases. The mucinous lesions are confined to the ovary in 95% to 98% of cases (Fig. 37.5). Because most ovarian mucinous carcinomas contain intestinal-type cells, they cannot be distinguished from metastatic carcinoma of the gastrointestinal tract on the basis of histology alone (2,6). Primary ovarian neoplasms rarely metastasize to the mucosa of the bowel, although they commonly involve the serosa, whereas gastrointestinal lesions frequently involve the ovary by direct extension or lymphatic spread (2).
Figure 37.4 High-grade serous adenocarcinoma. Papillae lined by sheets of cytologically malignant cells invade stroma, often with associated necrosis.
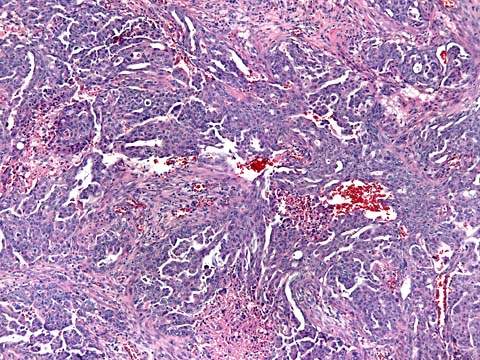
Figure 37.5 Mucinous adenocarcinoma of the ovary. Irregular glandular spaces are lined with a layer of tall columnar cells with abundant mucinous cytoplasm, resembling intestinal epithelium at the left. At the right, there is destructive invasion into the ovarian stroma.
Pseudomyxoma Peritonei
Pseudomyxoma peritonei is a clinical term used to describe the finding of abundant mucoid or gelatinous material in the pelvis and abdominal cavity surrounded by fibrous tissue. It is most commonly secondary to a well-differentiated appendiceal mucinous neoplasm or other gastrointestinal primary; rarely, mucinous tumors arising in an ovarian mature teratoma are associated with pseudomyxoma peritonei.
Endometrioid Tumors
Endometrioid lesions constitute 6% to 8% of epithelial tumors. Endometrioid neoplasia includes all the benign demonstrations of endometriosis. In 1925, Sampson suggested that certain cases of adenocarcinoma of the ovary probably arose in areas of endometriosis (19). The adenocarcinomas are similar to those seen in the uterine corpus. The malignant potential of endometriosis is very low, although a transition from benign to malignant epithelium may be demonstrated.
Borderline Endometrioid Tumors
The endometrioid tumor of low malignant potential has a wide morphologic spectrum. Tumors may resemble an endometrial polyp or complex endometrial hyperplasia with glandular crowding. When there are back-to-back, architecturally complex glands with no intervening stroma, the tumor is classified as a well-differentiated endometrioid carcinoma. Some borderline endometrioid tumors have a prominent fibromatous component. In such cases, the term adenofibroma is used to describe them (2).
Endometrioid Carcinomas
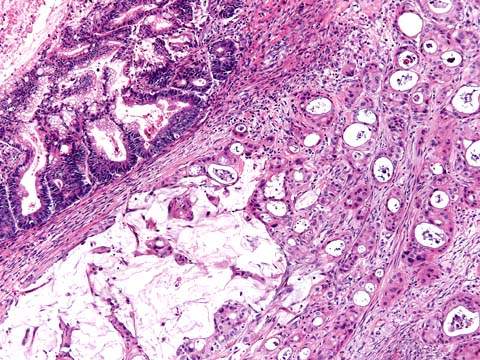
Endometrioid tumors are characterized by a markedly complex glandular pattern with all the potential variations of epithelia found in the uterus (Fig. 37.6).
Figure 37.6 Endometrioid cancer. Round to tubular glands lined by stratified columnar cells with confluent growth pattern.
Multifocal Disease
The endometrioid tumors afford the greatest opportunity to evaluate multifocal disease. Endometrioid carcinoma of the ovary is associated in 15% to 20% of the cases with carcinoma of the endometrium. Identification of multifocal disease is important because patients with disease metastatic from the uterus to the ovaries have a 30% to 40% 5-year survival, whereas those with synchronous multifocal disease have a 75% to 80% 5-year survival (20). When the histologic appearance of endometrial and ovarian tumors is different, the two tumors most likely represent two separate primary lesions. When they appear similar, the endometrial tumor can be considered a separate primary tumor if it is well differentiated and only superficially invasive.
Clear Cell Carcinomas
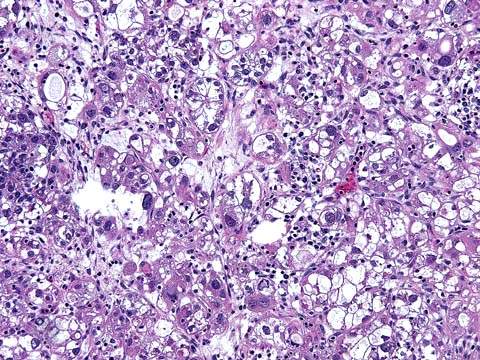
Several basic histologic patterns are present in the clear cell adenocarcinoma (i.e., tubulocystic, papillary, reticular, and solid). The tumors are made up of clear and hobnail cells that project their nuclei into the apical cytoplasm. The clear cells have abundant clear or vacuolated cytoplasm, hyperchromatic irregular nuclei, and nucleoli of various sizes (Fig. 37.7). Focal areas of endometriosis are common and mixed clear cell and endometrioid carcinoma may occur (20). The clear cell carcinoma seen in the ovary is histologically identical to that seen in the uterus or vagina of the young patient who has been exposed to diethylstilbestrol (DES) in utero. Nuclei of clear cell carcinoma range from grade 1 to grade 3, but pure grade 1 tumors are extremely rare. Almost invariably high-grade (grade 3) nuclei are identified. Hence, clear cell carcinoma is not graded.
Figure 37.7 Clear cell carcinoma of the ovary. Note the solid variant of clear cell carcinoma with sheets of cells that have clear cytoplasm (“hobnail” cells).
Transitional (Brenner) Tumors
Borderline Brenner Tumors
In the past, proliferative Brenner tumors were subclassified as proliferating tumors (those tumors that resemble low-grade papillary urothelial carcinoma of the urinary bladder) and borderline tumors (those tumors that resemble high-grade papillary urothelial carcinoma), but both groups of tumors are now classified as borderline Brenner tumors (21). Complete surgical removal usually results in cure.
Malignant Brenner Tumors
These rare tumors are defined as benign or borderline Brenner tumors coexisting with invasive transitional cell carcinoma.
Transitional Cell Carcinoma
The designation transitional cell carcinoma refers to a primary ovarian carcinoma resembling transitional cell carcinoma of the urinary bladder without a recognizable Brenner tumor. It is reported that those ovarian carcinomas that contain more than 50% of transitional cell carcinoma are more sensitive to chemotherapy and have a more favorable prognosis than other poorly differentiated ovarian carcinomas of comparable stage (22,23). Transitional cell tumors differ from malignant Brenner tumors in that they are more frequently diagnosed in an advanced stage and are associated with a poorer survival rate (24).
Peritoneal Carcinomas
Peritoneal tumors are histologically indistinguishable from ovarian serous tumors. In the case of borderline serous peritoneal tumors and serous peritoneal carcinomas, the ovaries are normal or minimally involved, and the tumors affect predominantly the uterosacral ligaments, pelvic peritoneum, or omentum. The overall prognosis for borderline serous peritoneal tumors is excellent and comparable to that of ovarian borderline serous tumors (25–27). In the review of 38 cases of peritoneal borderline serous tumors from the literature, 32 women had no persistent disease, 4 were well after resection of recurrence, 1 developed an invasive serous carcinoma, and 1 died from the effects of the tumor (25).
Carcinoma that appears predominantly as peritoneal carcinomatosis without appreciable ovarian or fallopian tube enlargement is called peritoneal carcinoma or müllerian carcinoma when tumors spread from the breast, gastrointestinal tract, and other organs of nonmüllerian origin are excluded. Most are peritoneal serous carcinomas, which have the appearance of a moderately to poorly differentiated serous ovarian carcinoma. Peritoneal endometrioid carcinoma is less common.
Peritoneal carcinoma should be considered clinically the same as ovarian and fallopian tube cancers. In patients for whom exploratory surgery is performed, there may be microscopic or small macroscopic cancer on the surface of the ovary and extensive disease in the upper abdomen, particularly in the omentum (28).
Mesotheliomas
Peritoneal malignant mesotheliomas may be epithelial, sarcomatous, or biphasic (2,29). Deciduoid peritoneal mesothelioma is an unusual variant that resembles exuberant, ectopic decidual reaction of the peritoneum. Asbestos exposure is not correlated with peritoneal mesotheliomas in women. These lesions typically appear as multiple intraperitoneal masses, often coating the entire peritoneum and can develop after hysterectomy and bilateral salpingo-oophorectomy for benign disease. Malignant mesotheliomas should be distinguished from benign multicystic peritoneal mesothelioma (multilocular peritoneal inclusion cyst), and ovarian tumor implants and primary peritoneal müllerian neoplasms.
Clinical Features
More than 80% of epithelial ovarian cancers are found in postmenopausal women (Fig. 37.8). The peak incidence of invasive epithelial ovarian cancer is at 56 to 60 years of age (2,3,30). The age-specific incidence of ovarian epithelial cancer rises precipitously from 20 to 80 years of age and subsequently declines (30). These cancers are relatively uncommon in women younger than age 45. Fewer than 1% of epithelial ovarian cancers occur before the age of 21 years, two-thirds of ovarian malignancies in such patients being germ cell tumors (2,30,31). About 30% of ovarian neoplasms in postmenopausal women are malignant, whereas only about 7% of ovarian epithelial tumors in premenopausal patients are frankly malignant (2,3).
Figure 37.8 Ovarian cancer incidence: distribution by age. (From Nagy K. The side effects of managed care on the drug industry. J Natl Cancer Inst 1995;87:1280, with permission.)
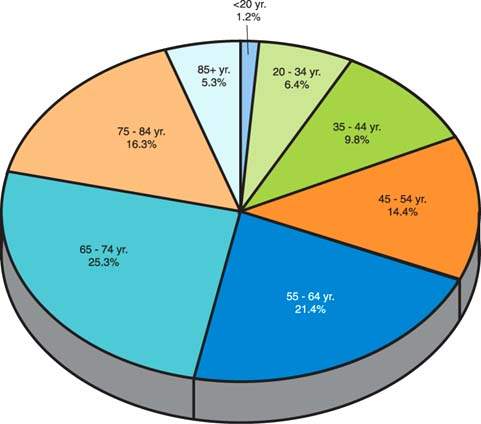
The average age of patients with borderline tumors is approximately 46 years (2,3,9). Eighty percent to 90% of ovarian cancers, including borderline forms, occur after the age of 40 years, whereas 30% to 40% of malignancies occur after the age of 65 years. The chance that a primary epithelial tumor will be of borderline or invasive malignancy in a patient younger than 40 years is approximately 1 in 10, but after that age it rises to 1 in 3 (2,3). Less than 1% of epithelial ovarian cancers occur before the age of 20 years, with two-thirds of ovarian malignancies in such patients being germ cell tumors (31).
Etiology
Ovarian cancer is associated with low parity and infertility (32). Although there are a variety of epidemiologic variables correlated with ovarian cancer, such as talc use, galactose consumption, and tubal ligation (see Chapter 4), none is so strongly correlated as prior reproductive history and duration of the reproductive career (32,33). Early menarche and late menopause increase the risk of ovarian cancer (33). These factors and the relationship of parity and infertility to the risk of ovarian cancer led to the hypothesis that suppression of ovulation may be an important factor. Theoretically, the surface epithelium undergoes repetitive disruption and repair. It is thought that this process might lead to a higher probability of spontaneous mutations that can unmask germline mutations or otherwise lead to the oncogenic phenotype (see Chapter 6).
Prevention
Because parity is inversely related to the risk of ovarian cancer, having at least one child is protective for the disease, with a risk reduction of 0.3 to 0.4. Oral contraceptive use reduces the risk of epithelial ovarian cancer (32). Women who use oral contraceptives for 5 or more years reduce their relative risk to 0.5 (i.e., there is a 50% reduction in the likelihood of development of ovarian cancer). Women who had two children and used oral contraceptives for 5 or more years have a relative risk of ovarian cancer as low as 0.3, or a 70% reduction (34). The oral contraceptive pill is the only documented method of chemoprevention for ovarian cancer, and it should be recommended to women for this purpose. When counseling patients regarding birth control options, this important benefit of oral contraceptive use should be emphasized. This is important for women with a strong family history of ovarian cancer.
The performance of a prophylactic salpingo-oophorectomy significantly reduces, but does not totally eliminate, the risk of nonuterine pelvic cancers; because the entire peritoneum is at risk, peritoneal carcinomas can occur in 2% to 3% of women even after prophylactic bilateral salpingo-oophorectomy (25,28).
A thorough discussion of the risks and benefits of oophorectomy should be undertaken in premenopausal women who are undergoing a hysterectomy for benign disease, who do not carry germline mutations, and do not have a family history that suggests that they are at higher than average risk for ovarian cancer (35). The ovaries may provide protection from cardiovascular disease and osteoporosis, and long-term mortality may not be decreased by the performance of prophylactic oophorectomy in women at population risk of ovarian cancer (36).
Screening
The value of tumor markers and ultrasonography to screen for epithelial ovarian cancer is not established by prospective studies. Screening results with transabdominal ultrasonography are encouraging in postmenopausal women, but specificity is limited (37–39). Advances in transvaginal ultrasonography showed a very high (>95%) sensitivity for the detection of early-stage ovarian cancer, although this test alone might require performance of as many as 10 to 15 laparotomy procedures for each case of ovarian cancer detected (37,38). Routine annual pelvic examinations have disappointing results in the early detection of ovarian cancer (40). Transvaginal color flow Doppler to assess the vascularity of the ovarian vessels is a useful adjunct to ultrasonography, but it is not useful in screening (41,42).
CA125 is useful for monitoring epithelial ovarian cancer patients during their chemotherapy, but the role of CA125 is still being defined in a screening setting (43–49). Regarding the sensitivity of the test, elevated CA125 levels are seen in 50% of patients with stage I disease (43,48). Data suggest that the specificity of CA125 is improved when the test is combined with transvaginal ultrasonography or when the CA125 levels are followed over time (49,50). These data encouraged the development of prospective screening studies in Sweden and the United Kingdom (45,47). In these studies, patients with elevated CA125 levels (>30 U/mL) underwent abdominal ultrasonography, and 14 ovarian cancers were discovered among 27,000 women screened. About four laparotomies were performed for each case of cancer detected (47).
A randomized trial of nearly 22,000 women aged 45 years or older was performed in the United Kingdom (50). The patients were assigned to either a control group of routine pelvic examination (n = 0,977) or to a screening group (n = 10,958). The screening consisted of three annual screens that involved measurement of serum CA125 levels, pelvic ultrasonography if the CA125 was 30 U/mL or higher, and referral for gynecologic examination if the ovarian volume was 8.8 mL or greater on the ultrasonography. Of the 468 women in the screened group with an elevated CA125, 29 were referred for surgery, 6 cancers were discovered, and 23 had false-positive screening results, yielding a positive predictive value of 20.7%. During a 7-year follow-up period, cancer developed in 10 additional women in the screened group, as it did in 20 women in the control group. Although the median survival of women in whom cancer developed in the screened group was 72.9 months, compared with 41.8 months in the control group (p = .0112), the number of deaths did not differ significantly between the control and screened groups (18/10,977 vs. 9/10,958; relative risk 2.0 [0.78 to 5.13]). These data show that a multimodal approach to ovarian cancer screening is feasible, but a larger trial is necessary to determine whether this approach affects mortality. Such a three-arm randomized trial is ongoing in the United Kingdom, and the anticipated accrual is approximately 50,000 women per study arm and 100,000 women in the control arm. Based on the risk of ovarian cancer (ROC) algorithm for CA125 levels, patients in the third group will be referred for transvaginal ultrasonography and/or surgery (51). Women will be screened for 3 years and studied for 7 years. The aims of this trial are to determine the feasibility of screening for ovarian cancer and whether ovarian cancers can be diagnosed at an earlier stage and the impact of early detection on survival.
Another approach is the use of proteomic patterns to identify ovarian cancer using surface-enhanced laser desorption ionization time-of-flight (SELDI-TOF) technology (52). In a study using this technology, the sensitivity for predicting ovarian cancer was 100%, with a specificity of 95% and a positive predictive value of 94%. The assay correctly identified all 18 women with stage I tumors. This technology is in the early phases of development and validation, and its efficacy has yet to be demonstrated in large population-based studies (53).
Given the false-positive and false-negative results for both CA125 and transvaginal ultrasonography and the absence of good data to show that screening detects ovarian cancers at an earlier stage, these tests are not recommended and should not be used routinely to screen women with a population risk or high risk for ovarian cancer (54–56). In the future, new markers or technologies may improve the specificity of ovarian cancer screening, but proof of this will require a large, prospective study (47,48). Screening in women who have a familial risk may have a better yield, but to date there is no evidence to demonstrate a benefit of screening even in high-risk women, and this is being actively investigated (55,57). The findings of two prospective studies of annual transvaginal ultrasound and CA125 screening in 888 BRCA1and BRCA2 mutation carriers in the Netherlands and 279 mutation carriers in the United Kingdom are not encouraging and suggest a very limited benefit of screening in high-risk women (55,56). Despite annual gynecologic screening Hermsen et al. reported that a high proportion of ovarian cancers in BRCA1-2 carriers were interval cancers and the large majority of all cancers diagnosed were at advanced stages; similar results were reported by Woodward et al. (55,56).
Genetic Risk for Epithelial Ovarian Cancer
The lifetime risk of ovarian carcinoma for women in the United States is about 1.4% (1–3). The risk of ovarian cancer is higher than that in the general population in women with certain family histories (51–60). Most epithelial ovarian cancer is sporadic, with familial or hereditary causes accounting for 5% to 10% of invasive epithelial ovarian cancer (59).
Hereditary Ovarian Cancer
BRCA1 and BRCA2
Most hereditary ovarian cancer is associated with mutations in the BRCA1 gene, located on chromosome 17 (58–69). A small proportion of inherited disease is associated with germline mutations in another gene, BRCA2, located on chromosome 13 (60). Discovered through linkage analyses, these two high-penetrance genes are associated with the genetic predisposition to both ovarian and breast cancers. There are almost certainly other low- to moderate-penetrance genes that predispose to ovarian and breast cancer, and this is an area of intense research interest (1).
It was thought that there were two distinct syndromes associated with a genetic risk, site-specific hereditary ovarian cancer and hereditary breast-ovarian cancer syndrome. It is now believed that these groups represent a continuum of mutations with different degrees of penetrance within a given family (62,70). There is a higher-than-expected risk of ovarian and endometrial cancer in Lynch syndrome, known as the hereditary nonpolyposis colorectal cancer syndrome (HNPCC syndrome) (71).
The mutations are inherited in an autosomal dominant fashion, and therefore a full pedigree analysis (i.e., both maternal and paternal sides of the family) must be carefully evaluated (62). There are numerous distinct mutations that were identified on each of these genes, and the mutations have different degrees of penetrance that may account for the preponderance of either breast cancer, ovarian cancer, or both, in any given family. Based on analysis of women who have a mutation in the BRCA1 gene and are from high-risk families, the lifetime risk of ovarian cancer may be as high as 28% to 44%, and the risk was calculated to be as high as 27% for those women with a BRCA2 mutation (59,60,66–69). The risk of breast cancer in women with a BRCA1 or BRCA2 mutation may be as high as 56% to 87%.
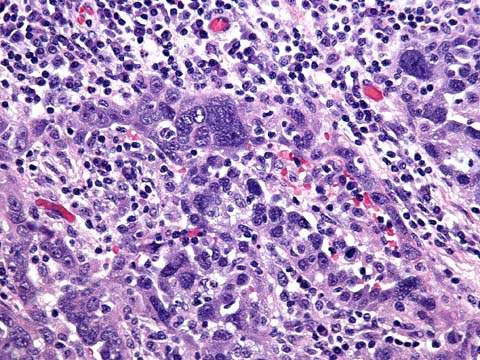
Hereditary ovarian cancers occur in women approximately 10 years younger than those with nonhereditary tumors (i.e., closer to age 50 compared to age 60 for those with sporadic cancer) (59). A woman with a first- or second-degree relative who had premenopausal ovarian cancer may have a higher probability of carrying an affected gene. Breast and ovarian cancer may exist in a family in which there is a combination of epithelial ovarian and breast cancers, affecting a mixture of first- and second-degree relatives. Women with this syndrome tend to have these tumors at a young age, and the breast cancers may be bilateral. If two first-degree relatives are affected, this pedigree is consistent with an autosomal dominant mode of inheritance (50,58). Most BRCA1 ovarian cancers are high-grade serous carcinomas (Fig. 37.9).
Figure 37.9 BRCA1-associated ovarian carcinoma is typically a high-grade serous adenocarcinoma with numerous mitotic figures and marked nuclear pleomorphism. A brisk lymphocytic infiltrate with tumor infiltrating lymphocytes is not uncommon in these tumors.
Founder Effect
There is a higher carrier rate of BRCA1 and BRCA2 mutations in women of Ashkenazi Jewish descent, in Icelandic women, and in many other ethnic groups (64,65,67–69). There are three specific founder mutations carried by the Ashkenazi population, 185delAG and 5382insC on BRCA1, and 6174delT on BRCA2. Individuals of Ashkenazi Jewish descent have a 1 in 40, or 2.5%, chance of having a mutation in BRCA1 or BRCA2, and thus there is a greater risk in this population. The increased risk is a result of the founder effect, in which a higher rate of specific mutations occurs in an ethnic group from a defined geographic area. These founder mutations generated considerable interest, because they facilitate studies of prevalence and penetrance and can be used to quantify the degree of homogeneity within a population.
Pedigree Analysis
The risk of carrying a germline mutation that predisposes to ovarian cancer depends on the number of first- or second-degree relatives (or both) with a history of epithelial ovarian carcinoma or breast cancer (or both) and on the number of malignancies that occurs at an earlier age. The degree of risk is difficult to determine precisely unless a full pedigree analysis is performed.
Lynch Syndrome or Hereditary Nonpolyposis Colon Cancer
Lynch syndrome (HNPCC), which includes multiple adenocarcinomas, involves a combination of colon cancer and endometrial or ovarian cancer and other malignancies of the gastrointestinal and genitourinary systems (71). The mutations that are associated with this syndrome are MSH2, MLH1, PMS1, and PMS2. The risk that a woman who is a member of one of these families will develop epithelial ovarian cancer depends on the frequency of this disease in first- and second-degree relatives, although these women appear to have at least three times the relative risk of the general population. A full pedigree analysis of such families should be performed by a geneticist to more accurately determine the risk.
Management of Women at High Risk for Ovarian Cancer
The management of a woman with a strong family history of epithelial ovarian cancer must be individualized and depends on her age, her reproductive plans, and the extent of risk. In all of these syndromes, women at risk benefit from a thorough pedigree analysis. A geneticist should evaluate the family pedigree for at least three generations. Decisions about management are best made after careful study and, whenever possible, verification of the histologic diagnosis of the family members’ ovarian cancer.
The value of testing for BRCA1 and BRCA2 is established, and there are guidelines for testing (62,70,72). The importance of genetic counseling cannot be overemphasized because the decision is complex. The American Society of Clinical Oncology offered guidelines that emphasize careful evaluation by geneticists, careful maintenance of medical records, and an understanding in a genetic screening clinic of how to effectively counsel and manage these patients. Concerns remain over the use of the information, the impact on insurability, the interpretation of the results, and how the information will be used within a specific family (e.g., to counsel children).
Although there are some conflicting data, the behavior of breast cancers arising in women with germline mutations in BRCA1 or BRCA2 is comparable to the behavior of sporadic tumors (61,73). Women with breast cancer who carry these mutations are at a greatly increased risk of ovarian cancer and a second breast cancer: the lifetime risk of ovarian cancer is 54% for women who have a BRCA1 mutation and 23% for those with a BRCA2 mutation, and for the two groups together, there is an 82% lifetime risk of breast cancer (73).
Despite recommendation by the National Institutes of Health Consensus Conference on Ovarian Cancer, the value of screening with transvaginal ultrasonography, CA125 levels, or other procedures is not established in women at high risk (74). Bourne and coworkers showed that, using this approach, tumors can be detected approximately 10 times more often than in the general population, and they recommend screening in high-risk women, but other groups have not confirmed these findings, and bilateral salpingo-oophorectomy remains the most effective way to reduce risk (57,75).
Data derived from a multicenter consortium of genetic screening centers indicate that the use of the oral contraceptive pill is associated with a lower risk for development of ovarian cancer in women who have a mutation in either BRCA1 or BRCA2 (76). The risk reduction is significant: in women who take oral contraceptives for 5 or more years, the relative risk of ovarian cancer is 0.4, or a 60% reduction in the incidence of the disease.
Prophylactic Salpingo-oophorectomy in High-Risk Women
The value of prophylactic salpingo-oophorectomy in these patients is documented (77–83). Women at high risk for ovarian cancer who undergo prophylactic salpingo-oophorectomy have a risk of harboring occult neoplasia: in one series of 98 such operations, 3 (3.1%) patients had a low-stage ovarian malignancy (80). The protection against ovarian cancer is excellent: the performance of a prophylactic salpingo-oophorectomy reduced the risk of BRCA–related gynecologic cancer by 96% (80). In a series of 42 such operations, 4 patients (9.5%) had a malignancy, 1 of which was noted at surgery and 3 that were microscopic; all were smaller than 5 mm (78). Although the risk of ovarian cancer is significantly diminished, there remains the small risk of peritoneal carcinoma, a tumor for which women who have mutations in BRCA1 and BRCA2 may have a higher predisposition. In these series, the subsequent development of peritoneal carcinoma was 0.8% and 1%, respectively (78,79). The risk of developing subsequent breast cancer was reduced by 50% to 80%.
The role of hysterectomy is more controversial. Most studies show no increase in the rate of uterine and cervical tumors, but there are rare reports of an increase of papillary serous tumors of the endometrium (83). Women on tamoxifen are at higher risk for benign endometrial lesions (e.g., polyps) and endometrial cancer. It is reasonable to consider the performance of a prophylactic hysterectomy in conjunction with salpingo-oophorectomy, but this decision should be individualized.
The survival of women who have a BRCA1 or BRCA2 mutation and develop ovarian cancer is longer than that for those who do not have a mutation. In one study, the median survival for mutation carriers was 53.4 months compared with 37.8 months for those with sporadic ovarian cancer from the same institution (84).
Recommendations
Current recommendations for management of women at high risk for ovarian cancer are summarized as follows (72,82):
Symptoms
The majority of women with epithelial ovarian cancer have vague and nonspecific symptoms (3,85–87). In early-stage disease, if the patient is premenopausal, she may experience irregular menses. If a pelvic mass is compressing the bladder or rectum, she may report urinary frequency or constipation (85–87). Occasionally, she may perceive lower abdominal distention, pressure, or pain, such as dyspareunia. Acute symptoms, such as pain secondary to rupture or torsion, are unusual.
In advanced-stage disease, patients have symptoms related to the presence of ascites, omental metastases, or bowel metastases. The symptoms include abdominal distention, bloating, constipation, nausea, anorexia, or early satiety. Premenopausal women may report irregular or heavy menses, whereas vaginal bleeding may occur in postmenopausal women (86).
Traditionally, ovarian cancer was considered a “silent killer” that did not produce symptoms until far advanced. Some patients with ovarian cancers confined to the ovary are asymptomatic, but the majority will have nonspecific symptoms that do not necessarily suggest an origin in the ovary (86,88–90). In one survey of 1,725 with ovarian cancer, 95% recalled symptoms before diagnosis, including 89% with stage I and II disease and 97% with stages III and IV disease (86). Some 70% had abdominal or gastrointestinal symptoms, 58% pain, 34% urinary symptoms, and 26% pelvic discomfort. At least some of these symptoms could have reflected pressure on the pelvic viscera from the enlarging ovary. Goff et al. developed an ovarian cancer symptom index and reported that symptoms associated with ovarian cancer, when present for less than 1 year and occurring longer than 12 days a month, were pelvic/abdominal pain, urinary frequency/urgency, increased abdominal size or bloating, and difficulty eating or feeing full (88). The index had a sensitivity of 56.7% for early ovarian cancer and 79.5% for advanced stage disease. A population-based study from Australia found that there did not appear to be a significant difference in the duration of symptoms or the nature of symptoms in patients with early as opposed to advanced stage ovarian cancer, reinforcing the concept that they are biologically different entities and arguing against the widely held misconception that early stage ovarian cancers are at an early stage because they were diagnosed earlier than patients with more advanced stage cancers (89).
Signs
The most important sign of epithelial ovarian cancer is the presence of a pelvic mass on physical examination. A solid, irregular, fixed pelvic mass is highly suggestive of an ovarian malignancy. If an upper abdominal mass or ascites is present, the diagnosis of ovarian cancer is almost certain. Because the patient usually reports abdominal symptoms, she may not have a pelvic examination, and a tumor may be missed.
In patients who are at least 1 year past menopause, the ovaries should be atrophic and not palpable. It was proposed that any palpable pelvic mass in these patients should be considered potentially malignant, a situation that was referred to as the postmenopausal palpable ovary syndrome (91). This concept was challenged, because subsequent authors reported that only about 3% of palpable masses measuring less than 5 cm in postmenopausal women are malignant (57).
Diagnosis
Ovarian epithelial cancers must be differentiated from benign neoplasms and functional cysts of the ovaries. A variety of benign conditions of the reproductive tract, such as pelvic inflammatory disease, endometriosis, and pedunculated uterine leiomyomas, can simulate ovarian cancer. Nongynecologic causes of a pelvic tumor, such as an inflammatory (e.g., diverticular) disease or neoplastic colonic mass, must be excluded (3). A pelvic kidney can simulate ovarian cancer.
Serum CA125 levels are useful in distinguishing malignant from benign pelvic masses (92). For a postmenopausal patient with an adnexal mass and a very high serum CA125 level (>200 U/mL), there is a 96% positive predictive value for malignancy. For premenopausal patients, the specificity of the test is low because the CA125 level tends to be elevated in common benign conditions.
For the premenopausal patient, a period of observation is reasonable provided the adnexal mass does not have characteristics that suggest malignancy (i.e., it is mobile, mostly cystic, unilateral, and of regular contour). An interval of no more than 2 months is allowed, during which hormonal suppression with an oral contraceptive may be used. If the lesion is not neoplastic, it should regress, as measured by pelvic examination and pelvic ultrasonography. If the mass does not regress or if it increases in size, it must be presumed to be neoplastic and must be removed surgically.
The size of the lesion is important. If a cystic mass is greater than 8 cm in diameter, the probability is high that the lesion is neoplastic, unless the patient is taking clomiphene citrate or other agents to induce ovulation (37–40). Premenopausal patients whose lesions are clinically suspicious (i.e., large, predominantly solid, relatively fixed, or irregularly shaped) should undergo laparotomy, as should postmenopausal patients with complex adnexal masses of any size.
Ultrasonographic signs of malignancy include an adnexal pelvic mass with areas of complexity, such as irregular borders, multiple echogenic patterns within the mass, and dense multiple irregular septae. Bilateral tumors are more likely to be malignant, although the individual characteristics of the lesions are of greater significance. Transvaginal ultrasonography may have a somewhat better resolution than transabdominal ultrasonography for adnexal neoplasms (93–96). Doppler color flow imaging may enhance the specificity of ultrasonography for demonstrating findings consistent with malignancy (97–99).
In postmenopausal women with unilocular cysts measuring 8 to 10 cm or less and normal serial CA125 levels, expectant management is acceptable, and this approach may decrease the number of surgical interventions (100–102).
The diagnosis of an ovarian cancer requires an exploratory laparotomy. The preoperative evaluation of the patient with an adnexal mass is outlined in Figure 14.19 (see Chapter 14).
Before the planned exploration, the patient should undergo routine hematologic and biochemical assessments. A preoperative evaluation in a patient undergoing laparotomy should include a radiograph of the chest. Abdominal and pelvic computed tomography (CT) or MRI are of limited value for a patient with a definite pelvic mass (103–105). A CT or MRI should be performed for patients with ascites and no pelvic mass to look for liver or pancreatic tumors. The findings only rarely preclude laparotomy (103). The value of PET scanning is still being evaluated (105–107). If the hepatic enzyme values are normal, the likelihood of liver disease is low. Liver-spleen scans, bone scans, and brain scans are unnecessary unless symptoms or signs suggest metastases to these sites.
The preoperative evaluation should exclude other primary cancers metastatic to the ovary. A barium enema or colonoscopy is indicated in selected patients with symptoms and signs suspicious for colon cancer. This study should be performed for any patient who has evidence of occult blood in the stool or of intestinal obstruction. An upper gastrointestinal radiographic series or gastroscopy is indicated if there are upper gastrointestinal symptoms such as nausea, vomiting, or hematemesis (3,108). Bilateral mammography is indicated if there is any breast mass, because breast cancer metastatic to the ovaries can simulate primary ovarian cancer.
A Papanicolaou (Pap) test should be performed, although its value for the detection of ovarian cancer is very limited. Patients who have irregular menses or postmenopausal vaginal bleeding should have endometrial biopsy and endocervical curettage to exclude the presence of uterine or endocervical cancer metastatic to the ovary.
Differential Diagnosis
Ovarian epithelial cancers must be differentiated from benign neoplasms and functional cysts of the ovaries (100–102). A variety of benign conditions of the reproductive tract, such as pelvic inflammatory disease, endometriosis, and pedunculated uterine leiomyomata, can simulate ovarian cancer. Nongynecologic causes of a pelvic tumor, such as an inflammatory or neoplastic colonic mass, must be excluded. A pelvic kidney can simulate ovarian cancer.
Patterns of Spread
Ovarian epithelial cancers spread primarily by exfoliation of cells into the peritoneal cavity, by lymphatic dissemination, and by hematogenous spread.
Transcoelomic
The most common and earliest mode of dissemination of ovarian epithelial cancer is by exfoliation of cells that implant along the surfaces of the peritoneal cavity. The cells tend to follow the circulatory path of the peritoneal fluid. The fluid moves with the forces of respiration from the pelvis, up the paracolic gutters, especially on the right, along the intestinal mesenteries, to the right hemidiaphragm. Metastases are typically seen on the posterior cul-de-sac, paracolic gutters, right hemidiaphragm, liver capsule, the peritoneal surfaces of the intestines and their mesenteries, and the omentum. The disease seldom invades the intestinal lumen but progressively agglutinates loops of bowel, leading to a functional intestinal obstruction. This condition is known as carcinomatous ileus (3).
Lymphatic
Lymphatic dissemination to the pelvic and para-aortic lymph nodes is common, particularly in advanced-stage disease (109–111). Spread through the lymphatic channels of the diaphragm and through the retroperitoneal lymph nodes can lead to dissemination above the diaphragm, especially to the supraclavicular lymph nodes (109). Burghardt et al. reported that 78% of patients with stage III disease have metastases to the pelvic lymph nodes (111). In another series, the rate of para-aortic lymph nodes positive for metastasis was 18% in stage I, 20% in stage II, 42% in stage III, and 67% in stage IV (109).
Hematogenous
Hematogenous dissemination at the time of diagnosis is uncommon. Spread to vital organ parenchyma, such as the lungs and liver, occurs in only about 2% to 3% of patients. Most patients with disease above the diaphragm when diagnosed have a right pleural effusion (3). Systemic metastases appear more frequently in patients who survived for some years. Dauplat et al. reported that distant metastasis consistent with stage IV disease ultimately occurred in 38% of the patients whose disease was originally intraperitoneal (112).
Prognostic Factors
The outcome of treatment can be evaluated in the context of prognostic factors, which can be grouped into pathologic, biologic, and clinical factors (113).
Pathologic Factors
The morphology and histologic pattern, including the architecture and grade of the lesion, are important prognostic variables (3). Histologic type was not believed to have prognostic significance, but several papers contained suggestions that clear cell carcinomas are associated with a prognosis worse than that of other histologic types (113,114).
Histologic grade, as determined either by the pattern of differentiation or by the extent of cellular anaplasia and the proportion of undifferentiated cells, seems to be of prognostic significance (115–118). Studies of the reproducibility of grading ovarian cancers show a high degree of intraobserver and interobserver variation (119,120). Because there is significant heterogeneity of tumors and observational bias, the value of histologic grade as an independent prognostic factor is not established. Baak et al. have presented a standard grading system based on morphometric analysis, and the system seems to correlate with prognosis, especially in its ability to distinguish low-grade or borderline patterns from other tumors (121).
Clinical Factors
In addition to stage, the extent of residual disease after primary surgery, the volume of ascites, patient age, and performance status are all independent prognostic variables (122–131). Among patients with stage I disease, Dembo et al. showed, in a multivariate analysis, that tumor grade and dense adherence to the pelvic peritoneum had a significant adverse impact on prognosis, whereas intraoperative tumor spillage or rupture did not (128). Sjövall et al. confirmed that ovarian cancers that undergo intraoperative rupture or spillage do not worsen prognosis, whereas tumors that are ruptured preoperatively do have a poorer prognosis (129). A multivariate analysis of these and several other studies was performed by Vergote et al., who found that for early-stage disease, poor prognostic variables were tumor grade, capsular penetration, surface excrescences, and malignant ascites, but not iatrogenic rupture (131).
Initial Surgery for Ovarian Cancer
Staging
Ovarian epithelial malignancies are staged according to the FIGO system listed in Table 37.2 (30). The FIGO staging is based on findings at surgical exploration. A preoperative evaluation should exclude the presence of extraperitoneal metastases.
Table 37.2 FIGO Staging for Primary Carcinoma of the Ovary
| Stage I | Growth limited to the ovaries. |
| IA | Growth limited to one ovary; no ascites containing malignant cells. |
| No tumor on the external surface; capsule intact. | |
| IB | Growth limited to both ovaries; no ascites containing malignant cells. |
| No tumor on the external surfaces; capsules intact. | |
| ICa | Tumor either stage IA or IB but with tumor on the surface of one or both ovaries; or with capsule ruptured; or with ascites present containing malignant cells or with positive peritoneal washings. |
| Stage II | Growth involving one or both ovaries with pelvic extension. |
| IIA | Extension and/or metastases to the uterus and/or fallopian tubes. |
| IIB | Extension to other pelvic tissues. |
| IICa | Tumor either stage IIA or IIB but with tumor on the surface of one or both ovaries; or with capsule(s) ruptured; or with ascites present containing malignant cells or with positive peritoneal washings. |
| Stage III | Tumor involving one or both ovaries with peritoneal implants outside the pelvis and/or positive retroperitoneal or inguinal nodes. Superficial liver metastasis equals stage III. Tumor is limited to the true pelvis, but with histologically proven malignant extension to small bowel or omentum. |
| IIIA | Tumor grossly limited to the true pelvis with negative nodes but with histologically confirmed microscopic seeding of abdominal peritoneal surfaces. |
| IIIB | Tumor of one or both ovaries with histologically confirmed implants of abdominal peritoneal surfaces, none exceeding 2 cm in diameter. Nodes negative. |
| IIIC | Abdominal implants >2 cm in diameter or positive retroperitoneal or inguinal nodes or both. |
| Stage IV | Growth involving one or both ovaries with distant metastasis. If pleural effusion is present, there must be positive cytologic test results to allot a case to stage IV. Parenchymal liver metastasis equals stage IV. |
These categories are based on findings at clinical examination or surgical exploration or both. The histologic characteristics are to beconsidered in the staging, as are results of cytologic testing as far as effusions are concerned. It is desirable that a biopsy be performed onsuspicious areas outside the pelvis. FIGO, International Federation of Obstetrics and Gynecology. aTo evaluate the impact on prognosis of the different criteria for allotting cases to stage IC or IIC, it would be of value to know if rupture of thecapsule was (i) spontaneous or (ii) caused by the surgeon and if the source of malignant cells detected was (i) peritoneal washings or (ii) ascites. Reproduced from Berek JS, Hacker NF, Berek & Hacker’s Gynecologic Oncology. 5th ed. Lippincott Williams & Wilkins. 2010:455, adaptedfrom FIGO Annual Report, Vol 26, Int J Gynecol Obstet 2006;105:3–4. | |
The importance of thorough surgical staging cannot be overemphasized, because subsequent treatment will be determined by the stage of disease. For patients in whom exploratory laparotomy does not reveal any macroscopic evidence of disease on inspection and palpation of the entire intra-abdominal space, a careful search for microscopic spread must be undertaken. In earlier series in which patients did not undergo careful surgical staging, the overall 5-year survival for patients with apparent stage I epithelial ovarian cancer was only about 60% (132). Since then, survival rates of 90% to 100% are reported for patients who were properly staged and were found to have stage IA or IB disease (133,134).
Technique for Surgical Staging
In patients whose preoperative evaluation suggests a probable malignancy, a midline or paramedian abdominal incision is recommended to allow adequate access to the upper abdomen (3,132). When a malignancy is unexpectedly discovered in a patient who has a lower transverse incision, the rectus muscles can be either divided or detached from the symphysis pubis to allow better access to the upper abdomen. If this is not sufficient, the incision can be extended on one side to create a “J” incision (3).
The ovarian tumor should be removed intact, if possible, and a frozen histologic section should be obtained. If ovarian malignancy is present and the tumor is apparently confined to the ovaries or the pelvis, thorough surgical staging should be performed. Staging involves the following steps (3,132):
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


