(1)
Department Obstetrics & Gynaecology, St George Hospital, Kogarah, New South Wales, Australia
Abstract
In the past, doctors recommended a particular treatment because, in their experience, most patients said that they were “better” after receiving it. In the last two decades, we have realized that this is not good enough. We need objective measures by which we can determine what percentage of patients are “cured” (normal) or at least have greater than 50 % reduction in symptoms, after any given treatment.
Introduction
In the past, doctors recommended a particular treatment because, in their experience, most patients said that they were “better” after receiving it. In the last two decades, we have realized that this is not good enough. We need objective measures by which we can determine what percentage of patients are “cured” (normal) or at least have greater than 50% reduction in symptoms, after any given treatment.
In this century, outcome measures are going to become even more important, because there is not enough money to fund all health care. Doctors (and administrators) must assess whether one treatment is more effective than another, so that money can be spent on that which is most effective. This is loosely termed “health economics.”
In the 1980s, continence clinicians began to realize the importance of outcome measures. It was a time of great creation. Many different outcome measures were created but not necessarily fully “validated.” The process of validation involves the following steps:
Establish the validity of the test that it measures what it is supposed to—includes three subsets: content validity, construct validity, and criterion validity.
Establish the reliability of the test. For questionnaires, measure internal consistency of different parts of test. For questionnaires and other physical tests, the reproducibility, or test–retest reliability, needs to be proven.
Establish the responsiveness to change of the test, before and after treatment.
This chapter provides a brief overview of outcome measures that have been validated. Most are used in this book to describe the effectiveness of different treatments.
The Standardization Committee of the International Continence Society (ICS) is the main body that has governed terminology and outcome measures in the field of urinary incontinence since 1978 [8]. The urodynamic measures described in the previous chapter and the pelvic floor assessments (Oxford score and POPQ) described in Chap. 2 are also used as outcome measures. The tests described in this chapter do not require physical examination or invasive procedures. More recently, the International Urogynecology Society (IUGA) has also created a joint ICS and IUGA standardization of terminology document which also fully covers the terminology for prolapse conditions. The IUGA website is http://www.iuga.org/.
The World Health Organization has recently acknowledged the global importance of incontinence, by holding a regular International Consultation on Incontinence (ICI) Abrams et al. [1–3]. These publications also consider which treatments are the most effective, as judged by standardized outcome measures. Finally, the Cochrane Collaboration performs meta-analyses of randomized controlled trials in the field of incontinence, which also use the outcome measures described in this text.
The ICS recommends that there should be five main groups, or “domains,” of outcome measures [9].
1.
Patient’s observations (symptoms)
2.
Quantification of symptoms (e.g., urine loss on diary or pad test)
3.
Physician’s observations (anatomical and functional)
4.
Quality of life measures
5.
Socioeconomic evaluations
Tests That Measure Patient’s Symptoms
The ICIQ-SF was validated under the auspices of the ICI. It records incontinence symptoms and severity, with a simple quality of life question. The final ICIQ comprises three scored items (Fig. 5.1, maximum score 21) and an unscored self-diagnostic item.
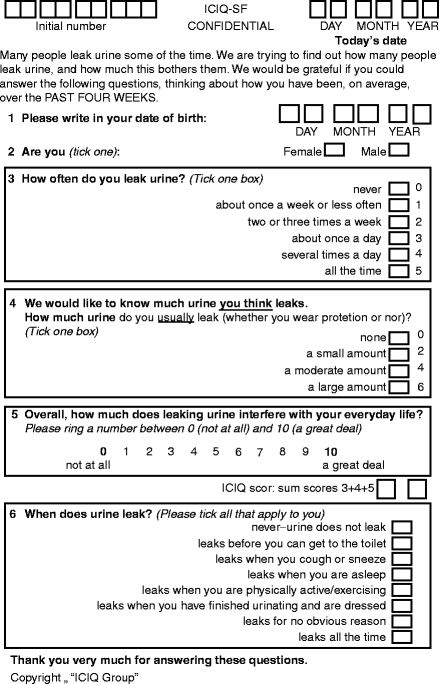

Figure 5.1
The ICIQ-SF questionnaire
The Wexner Score for Fecal Incontinence
This was originally a 20-point score concerning three types of incontinence with one question for impact upon lifestyle (italic bold in Table 5.1). Later, a score for wearing pads, taking constipating medication, or suffering from fecal urgency was added (ordinary typeface in Table 5.1). The Wexner score has been fully validated [15] and is used worldwide.
Table 5.1
Modified Wexner fecal incontinence score
Never | Rarely | Sometimes | Weekly | Daily | |
|---|---|---|---|---|---|
Incontinent solid stool | 0 | 1 | 2 | 3 | 4 |
Incontinent liquid stool | 0 | 1 | 2 | 3 | 4 |
Incontinent to gas | 0 | 1 | 2 | 3 | 4 |
Alters lifestyle | 0 | 1 | 2 | 3 | 4 |
No | Yes | ||||
Need to wear pad/plug | 0 | 2
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|