Unless the mother has overt TB, the diagnosis of perinatal TB is difficult and often delayed. The diagnosis of maternal TB has often been missed during the pregnancy and is only made when the infant presents unwell or if the mother deteriorates after delivery. Infants with signs and symptoms due to TB at birth are usually thought clinically to have a congenital TORCH (toxoplasmosis, rubella, cytomegalovirus, herpes and other) infection (see Chapter 17), congenital syphilis (Section 18.3) or possibly hemophagocytic lymphohistiocytoysis (HLH)11 (Section 18.4). Infants presenting in the second or third week of life are usually suspected initially as having late-onset bacterial infection.
Neonatal TB meningitis is mercifully rare, except in neonates with disseminated disease, but usually devastating. Lumbar puncture should be performed in newborns with proven or suspected disseminated TB disease.
18.1.4 Diagnosis
The diagnosis of perinatal tuberculosis is usually based on clinical grounds supported by chest radiology. It should be confirmed if possible by accredited laboratory tests including histopathology and/or microbiology testing. A chest radiograph should be performed in all infants with possible TB. Tuberculin skin testing and interferon-gamma release assays may be performed after an interval, but are often negative in neonatal infection, so are only helpful if positive. Respiratory secretions and gastric aspirates should be cultured for M. tuberculosis and tested by a polymerase chain reaction (PCR) test (such as Gene X-pert) if available. TB cultures and PCR can also be performed on CSF, pleural or ascitic fluid and blood. The placenta should be examined histopathologically and culture and PCR for M. tuberculosis performed. The CSF appearance of TB meningitis is of a lymphocytic pleocytosis with a high CSF protein, often >1 g/L (10 g/dL).12 The main differential diagnosis of this CSF finding is congenital toxoplasmosis (Section 18.2).
Accredited laboratory tests do not include antibody tests. Serologic antibody testing for M. tuberculosis is widely practiced in the private sector in India, at an estimated cost of US $15 million annually, despite strong evidence that serologic tests are unreliable13 and despite strong recommendations that they should not be used for diagnosis.14
18.1.5 Treatment
Treatment often needs to be started empirically prior to laboratory confirmation if there is a strong history of exposure to an infectious source and a typical clinical picture.
Infants from areas with a low prevalence of isoniazid resistance can be treated with three drugs (rifampin also called rifampicin in Europe, isoniazid and pyrazinamide) for 2 months, followed by rifampin/rifampicin plus isoniazid for a further 4 months if there is a good clinical response and/or the source or infant’s organism is fully drug susceptible.15,16 Infants with extensive infection and infants from areas with a high prevalence of isoniazid resistance should receive a fourth drug (ethambutol) during the 2-month intensive phase of treatment.9,10 Despite past concerns about retinal toxicity, the WHO advises that ethambutol is safe in children of all ages.15,16
The recommended doses16 are as follows:
| Isoniazid | 10 mg/kg once daily orally (range 7–15 mg/kg) |
| Rifampin (rifampicin) | 15 mg/kg once daily orally (range 10–20 mg/kg) |
| Pyrazinamide | 35 mg/kg once daily orally (range 30–40 mg/kg) |
| Ethambutol | 20 mg/kg once daily orally (range 15–25 mg/kg) |
The recommendations for treatment of multi-resistant TB are highly specialized, depending on sensitivities and available medications.17–20
The treatment of TB meningitis requires specialized advice, if available. It is usual to use the above four drugs if the organism is unlikely to be multi-resistant. Corticosteroids, either dexamethasone or prednisone, have been shown to reduce morbidity and mortality in older children and adults with tuberculous meningitis.21
18.1.6 Isolation
Recommendations on isolation of the infant and/or mother and separation of an infected mother from her infant need to be based on the risks and benefits. In contrast to adults or adolescents with open or cavitary pulmonary TB, children with primary TB are not usually infectious because the organism load is so much lower. However, infants with congenital TB often have extensive pulmonary involvement with large numbers of AFB.22 Transmission from infants with congenital TB to health-care workers has only ever been described once23 and never to other infants.23–39 Despite this low risk, it is recommended to isolate an infant with suspected congenital TB until it is known whether or not the infant is positive for AFB and also to isolate the mother and screen all relatives or visitors for signs and symptoms suggestive of TB. Infection control precautions and screening of exposed infants and staff should be reviewed in the light of whether or not infectious cases are identified (Section 18.1.7).
There is no evidence that a mother with suspected pulmonary TB needs separating from her infant, and it seems illogical if the mother is on adequate anti-tuberculous treatment and the baby is also receiving preventive therapy or treatment. Nevertheless such separation is sometimes recommended.22 Continued breastfeeding and development of the important mother–infant relationship should take priority in a situation where the neonate is under close scrutiny and receiving appropriate treatment.
18.1.7 Nursery exposure
If a member of staff on a neonatal nursery or a parent is diagnosed with sputum smear-positive cavitary pulmonary TB (sometimes loosely called ‘open TB’), there is understandable concern about exposure of vulnerable neonates and of other parents and staff. Such nursery exposures almost inevitably provoke a vigorous public health response with widespread tracing and testing of contacts, some of whom will have been exposed weeks or months earlier.23–39 Extensive epidemiologic investigations are usually recommended.22,36 The financial and emotional cost of these investigations is high and should be carefully weighed against the risk of potentially devastating disease. The infectiousness of the source case, the duration and the setting of exposure and the evidence from screening other contacts at higher risk may influence the assessment of risk.32 Important determinants of transmission risk in neonatal units include ventilation, room size, proximity to the index case, infectiousness of the index case and duration of contact.39 The key determinant influencing transmission is the duration of time spent in a room with the infectious source case.40
Exposed infants are recommended to have a clinical examination, a chest radiograph and consideration of prophylactic isoniazid and BCG vaccine. A tuberculin skin test (TST) is not usually performed immediately because of the delay in TST conversion following primary M. tuberculosis infection. It is, therefore, delayed until the infant is 3–4 months old. If the TST is negative at this point and the infant is clinically well and thriving, M. tuberculosis infection can be ruled out with a fair amount of certainty, chemoprophylaxis can be stopped and BCG vaccination considered.
Investigations of TB outbreaks are extremely time-consuming and costly and generate great anxiety. Prevention is ideal and screening of all staff for tuberculosis is an essential aspect of such prevention.
18.1.8 BCG immunization
Neonatal BCG vaccine will be considered in Section 23.5.1.
18.2 Toxoplasmosis
Congenital toxoplasmosis is a potentially devastating condition that arguably is preventable and the outcome can be improved if diagnosed and treated early.
18.2.1 Epidemiology
Toxoplasmosis is a zoonosis and the cat is the definitive host. The worldwide incidence of toxoplasmosis and congenital toxoplasmosis varies enormously and is an important determinant of the most appropriate detection, prevention and treatment strategies.41 Factors affecting the incidence of toxoplasmosis include exposure to cats and particularly cat faeces, undercooked meat and climatic conditions, which affect oocyst maturation in soil.41
18.2.2 Microbiology
Toxoplasma gondii is a coccidian parasite. Its main host is the cat where it lives in the intestine. Outside the cat intestine it can exist as an oocyst, in which sporozoites form, as a trophozoite (also called an endozoite or tachyzoite) and as a tissue cyst. It is related to Cryptosporidium and Plasmodium.
Congenital toxoplasmosis follows primary maternal infection. Women infected with T. gondii in the first trimester have a relatively low risk (14%) of congenital toxoplasmosis; 6% of their infants are severely affected and 5% are perinatal deaths or stillbirths. In the second and third trimesters, the risk of congenital toxoplasmosis rises (to 29% and 59%, respectively), but the risk of severe disease falls. In the second trimester 2% of infants are severely affected and 2% are perinatal deaths or stillbirths, while babies infected in the third trimester are usually unaffected or sub-clinically infected and only 6% are mildly affected.41
18.2.3 Clinical
The classic triad of congenital toxoplasmosis is hydrocephalus (Figure 18.3), chorioretinitis (Figure 18.4) and brain calcification (Figure 18.3). In a US series of 164 infants with serologically confirmed congenital toxoplasmosis whose mothers had not been treated for the parasite during gestation, from 1991 to 2005, one or more severe clinical manifestations of congenital toxoplasmosis were reported in 84% of the infants. The commonest was eye disease (92.2%), followed by brain calcifications (79.6%) and hydrocephalus (67.7%). In 61.6% of the infants, eye disease, brain calcifications and hydrocephalus were present concurrently.42 The authors commented that their results contrasted markedly with European investigators who rarely report severe clinical signs in infants with congenital toxoplasmosis.42
Figure 18.3 Cerebral CT scan showing characteristic punctate calcification of toxoplasmosis and mild ventricular dilatation.
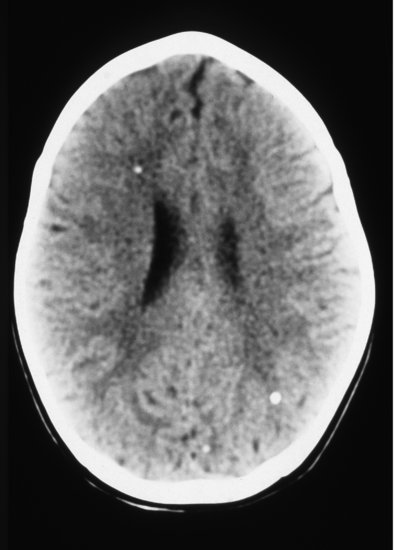
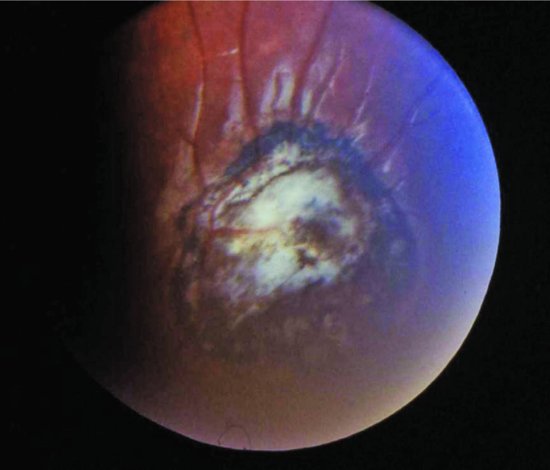
Figure 18.4 Toxoplasma choroidoretinitis showing typical white central area with surrounding pigmentation.
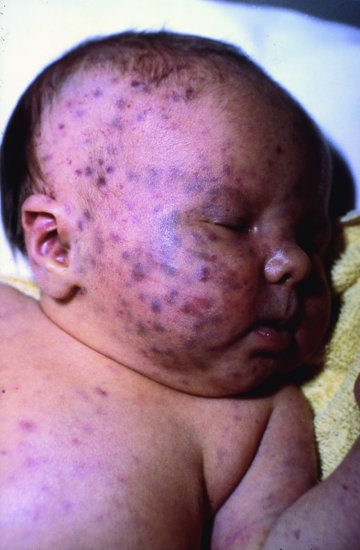
Other clinical signs and symptoms present in a minority of babies include a blueberry muffin rash present at birth due to extramedullary haemopoiesis in the skin (Figure 18.5), hepatosplenomegaly and jaundice, all of which may be seen with congenital rubella or congenital CMV infection. Indeed, there have been several case reports of congenital co-infection with T. gondii and CMV.41
Figure 18.5 One-day-old full-term infant with ‘blueberry muffin’ appearance, subsequently shown to have congenital toxoplasmosis.
Babies with congenital infection can be asymptomatic at birth. Other presentations include erythroblastosis and hydrops fetalis. Non-specific signs include fever, lymphadenopathy, vomiting, diarrhoea and pneumonitis. Although hydrocephalus is classical, microcephaly is described. The chorioretinitis is a focal necrotizing retinitis, with solitary or multiple yellow or white patches in the fundus with surrounding pigmentation (Figure 18.5). These may be seen at birth if there are other signs or suspicions that lead to a fundoscopic examination. However, it is not uncommon for presentation to be delayed until a few months of age when an infant develops visual signs such as strabismus (Figure 18.4). Rarer eye signs include glaucoma, optic atrophy and microphthalmia.41,42
In addition to the sites already mentioned, toxoplasma can occasionally disseminate and be found in almost any tissue in the body, including ears (deafness), heart (myocarditis), kidney (glomerulonephritis, nephrotic syndrome), adrenals, pancreas and thyroid (patchy necrosis) and skeletal muscle (myositis).41,42
18.2.4 Diagnosis
Clinical examination of the baby with suspected toxoplasmosis should include a full neurologic examination including fundoscopy. Investigations should include lumbar puncture. While CT scan is the radiologic investigation of choice, the radiation exposure has led some clinicians to be more conservative and restrict investigation to skull radiograph and cerebral ultrasound scan.
The definitive laboratory diagnosis of congenital toxoplasmosis is primarily serologic although nucleic acid amplification by PCR on CSF can add valuable confirmatory information. In the large US case series, T. gondii-specific IgM, IgA and IgE antibodies were demonstrated in 86.6%, 77.4% and 40.2% of the infants, respectively. Combined testing for both IgM and IgA antibodies increased the diagnostic sensitivity to 93% compared with testing for either IgM or IgA alone. IgM and IgA antibodies were still present in 44% of infants diagnosed between 1 and 6 months of life.42
The CSF is abnormal in most infants with congenital toxoplasmosis, classically showing xanthochromia, mononuclear cell pleocytosis and raised CSF protein which, though non-specific, may alert the clinician to request toxoplasma PCR. The CSF protein is often >2 g/L (20 g/dL), higher than in other infections except congenital TB. PCR can also be used to test amniotic fluid for antenatal diagnosis.35,37
18.2.5 Treatment
Authorities recommend 1 year of treatment with anti-parasitic drugs for symptomatic infants with congenital toxoplasmosis. There are no RCTs of duration of treatment of infants with congenital toxoplasmosis.
Remington41 recommends the following regimen, based on pharmacokinetic data:
- Pyrimethamine: Loading dose of 1 mg/kg 12 hourly for 2 days. From day 3, 1 mg/kg daily for 2–6 months, then 1 mg/kg three times a week to total 1 year.
- Sulfadiazine: 50 mg/kg 12 hourly for 1 year.
Remington also recommends folinic acid 10 mg three times weekly.41
There are no data on the adjunctive use of corticosteroids, but prednisone 0.5 mg/kg 12 hourly is sometimes prescribed for infants with CSF protein >10 g/L (1 g/dL) or with vision-threatening chorioretinitis.41
Remington41 recommends treating asymptomatic infants with congenital toxoplasmosis but the risks and benefits of such treatment are unknown.
18.2.6 Prevention
18.2.6.1 Primary prevention: education
Primary prevention consists of educating pregnant women to avoid risk activities such as changing the cat litter and eating undercooked meat.44 A Cochrane systematic review of RCTs and quasi- RCTs of all types of prenatal education on toxoplasmosis infection during pregnancy44 found one cluster-RCT (432 women) of poor methodological quality.
18.2.6.2 Secondary prevention: maternal screening and treatment
Maternal toxoplasmosis may be diagnosed if the mother presents with a ‘flu-like’ illness or by maternal screening. The major problem with the serologic diagnosis of toxoplasmosis is that IgM antibodies can persist for months or even years. For this reason, a single serum sample positive for toxoplasma IgM is difficult to interpret without prior serology. Amniocentesis to perform PCR on amniotic fluid and to test for IgM or IgA antibodies in foetal blood provides a more definitive diagnosis, but is associated with a small but not insignificant risk (up to 1%) of foetal loss.45
The rationale for maternal screening for toxoplasmosis is to offer the mother either termination of pregnancy or treatment. In countries where toxoplasmosis is common, maternal screening is often practiced. France began screening for congenital toxoplasmosis in 1978; from 1980 to 1995 the seroconversion rate in non-immune women during pregnancy was 4–5 per 1000.46 However, there is a significant psychological toll from screening45 and many women will terminate their pregnancy on the basis of a serologic result of uncertain significance.
Four non-Cochrane systematic reviews47–50 and one study of case series51 compared anti-parasitic treatment (spiramycin, pyrimethamine–sulphonamides or both) with no treatment. All four systematic reviews concluded that there are no satisfactory RCTs. The most recent GRADE review concluded that it is not certain whether treating infected pregnant women with spiramycin, pyrimethamine–sulphonamides or both reduces the risk of foetal infection, as the few trials they found produced conflicting results.47 The authors even suggested that treatment of infection in pregnancy might possibly save the pregnancy without preventing infection, which could increase the prevalence of congenital disease.47
A cost-effectiveness analysis based on US data found that screening and treatment of pregnant women was cost-saving for rates of congenital infection above 1 per 10 000 live births, if the parameters in the model were correct.52 However, since the best evidence cannot confirm that maternal treatment is beneficial, the assumptions in the model are highly questionable, to say the least.
In 2013, Canada made the following recommendations53: