Other important routes of osteomyelitis include direct inoculation and extension from adjacent structures. Calcaneal osteomyelitis can result from direct inoculation when heel pricks are performed on the point of the heel overlying the calcaneus (Figure 9.2) instead of the recommended area of the fleshy pads to the side of the heel10 (Figure 9.3). Other procedures that can cause osteomyelitis due to direct inoculation include scalp electrodes,11 femoral punctures, radial arterial punctures and rarely lumbar puncture. Fracture and osteomyelitis may coexist; in some cases there may be evidence of the fracture coming first12 but it may be difficult otherwise to distinguish pre-existing from pathologic fracture.
Figure 9.2 Abscess due to calcaneal osteomyelitis caused by a heel prick on the point of the heel overlying the os calcis, instead of the sides of the foot.
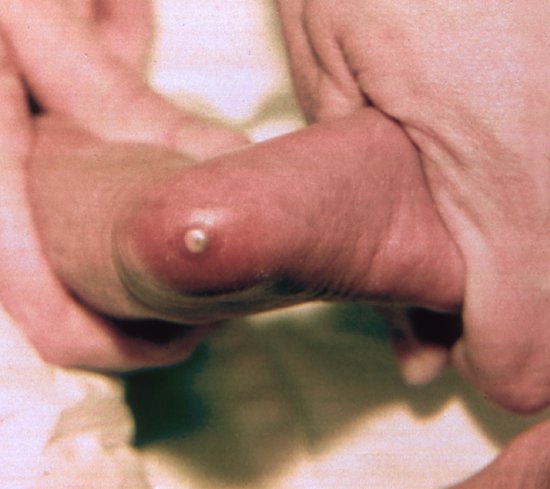
Figure 9.3 Correct site for heel pricks in fleshy part at side of foot (white shaded area). Reproduced with permission from Reference 9.
Skull osteomyelitis following infected cephalhaematoma13 and osteomyelitis of the phalanx following paronychia are examples of direct spread from adjacent structures.
Antenatal and perinatal complications are described in up to a half of infants in a case series of neonatal osteomyelitis.1
Intravascular catheters are a potential source of infection which can disseminate to bone, although neonatal osteomyelitis remains rare despite the frequent use of catheters and is surprisingly rare as a complication of central-line-associated bacteraemia.
Transplacental spread occurs in syphilis and infants with congenital syphilis may have osteitis (Figure 18.9). However, transplacental spread is otherwise thought to be a rare cause of neonatal osteomyelitis.
9.3 Clinical
It has been recognized for over 100 years that neonatal osteomyelitis has two clinical presentations: benign and severe. In the pre-antibiotic era, the severe form was common and there was a recognized association with omphalitis, but in recent times over 80% of patients have had a benign presentation.3,14-16
Neonates who present with the benign form of osteomyelitis often appear remarkably well. They may be afebrile and feed well. The most common presentation is with swelling, tenderness and decreased movement.3,14-16 Sometimes the neonate will not move a limb but otherwise does not seem to be in pain (pseudopalsy). If the immobile limb is an arm, the infant may be misdiagnosed as having Erb’s palsy (Figure 9.4) and if a leg, misdiagnosed as foot-drop from sciatic nerve injury (Figure 9.5a).16 Limb swelling may be due to an abscess from a ruptured bone abscess and can be apparently painless, at least at rest. Infants with the benign presentation have a low mortality, but may be left with bony complications. Progression to disseminated osteomyelitis has a worse prognosis for survival (Figure 9.5b).16 Early diagnosis is important to limit damage and avoid dissemination. The possibility of underlying osteomyelitis should always be considered in an infant with cellulitis, with or without swelling. Neonatal maxillary cellulitis is well recognized, for example, to be associated with underlying osteomyelitis.17 Similarly, limb swelling without discoloration or systemic symptoms should alert clinicians to possible osteomyelitis.
Figure 9.4 Baby with cleft lip and palate who stopped moving left arm. The Moro response (shown) was abnormal mimicking Erb’s palsy. There was swelling overlying the left clavicle, blood cultures grew S. aureus and the diagnosis was confirmed as osteomyelitis of the clavicle. Reproduced with permission from Reference 16.
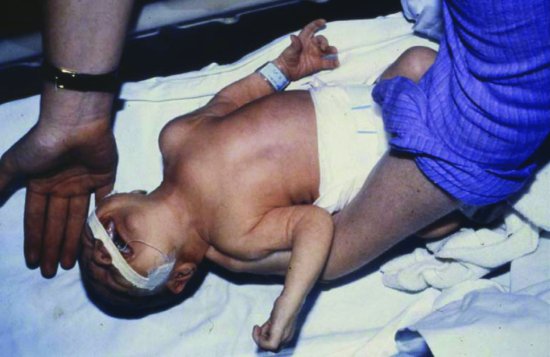
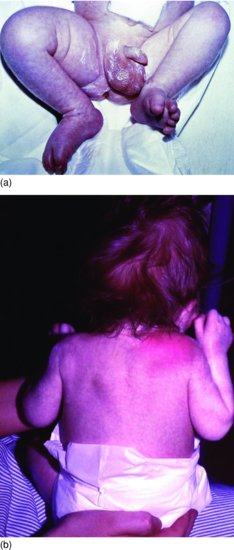
Figure 9.5 (a) Boy aged 2 weeks passed urine through umbilicus (patent urachus) and had foot-drop. Misdiagnosed as sciatic nerve damage from intramuscular injection. Note swelling of the right thigh.16 (b) Same baby as (a) developed cellulitis over shoulder and wrist. Blood cultures grew S. aureus. Swelling in thigh aspirated 30 mL of pus. Necrosis of femoral head of femur secondary to septic arthritis of hip. Diagnosis: multifocal staphylococcal osteomyelitis and septic arthritis.16
While the long bones are involved in over two-thirds of cases of neonatal osteomyelitis, almost any bone (and joint) can be involved. Neonatal vertebral osteomyelitis is notoriously difficult to diagnose, often not until an abscess ruptures through to the periphery or to the peritoneum.18 The clavicle and ribs are other well-recognized sites, while pelvic, scapular, sternal, mandibular and phalangeal osteomyelitis are all described.1 The joints associated with septic arthritis are classically those close to long bones, particularly hip, shoulder, knee, ankle and wrist joints.1,5,19-24
In a case series of 94 neonates and infants <;4 months old investigated for possible osteomyelitis, 30 babies were diagnosed with osteomyelitis or septic arthritis. Four (16%) of 27 babies investigated for S. aureus bacteraemia without focal signs or symptoms were diagnosed with osteomyelitis or septic arthritis by imaging.5
Routine bone scan and hip ultrasound scan should be considered for neonates who grow S. aureus in blood cultures, even in the absence of signs.
Between a third and a half of neonatal osteomyelitis is multifocal, including infants with a benign presentation.1-5,13-15 GBS osteomyelitis and/or septic arthritis classically presents with a benign presentation at 2–4 weeks of age only involving one limb, usually humerus (Figure 9.5) or femur.1,16,19 GBS osteomyelitis and arthritis are manifestations of late-onset infection and, like GBS meningitis and other forms of late-onset GBS disease, are usually caused by serotype III (Chapter 14). Early-onset GBS infection is virtually never associated with osteoarticular infection.1,19
Pre-term infants are more likely to present early than full-term infants: in a study from Australia 5 of 17 pre-term infants (29.4%) presented in the first week of life but only 1 of 13 full-term infants.5
In severe osteomyelitis, the infant has signs of sepsis with lethargy, irritability, feeding problems, vomiting and abdominal distension, with or without fever. Symptoms or signs of osteomyelitis may be present concurrently or may develop later, even after appropriate antibiotics have been started.3,15,16
9.4 Organisms
Staphylococcus aureus, by far the most common cause of neonatal osteomyelitis and septic arthritis in pre-antibiotic times, remains a major pathogen in Western and developing countries (Table 9.1).1-5 Since the 1970s, GBS sometimes outnumbered S. aureus in a case series from North America.1,4,20
Table 9.1 Organisms causing neonatal osteomyelitis and septic arthritis.
| Staphylococcus aureus (including MRSA) Group B streptococcus Gram-negative bacilli (increasingly common in developing countries)
Other Gram-positive cocci
Fungi, particularly Candida Neisseria gonorrhoeae Treponema pallidum Anaerobes |
In early reports of osteomyelitis, most S. aureus isolates were penicillin sensitive.1,3 Only about 10% of S. aureus are currently penicillin sensitive. Neonatal unit outbreaks of MRSA osteomyelitis and septic arthritis have been reported.25,26 Community-acquired neonatal MRSA osteoarticular infection is rarely reported.
Gram-negative bacilli are rare causes of neonatal osteoarticular infection in Western countries, but have been increasingly reported to cause neonatal Gram-negative osteomyelitis and septic arthritis in developing countries,23,27 including infections caused by multi-resistant organisms including extended spectrum beta-lactamase (ESBL) producers.13 Klebsiella predominates, but almost any Gram-negative bacillus has been reported at least once as causing osteoarticular infections (Table 9.1).1,23,27
Other organisms reported as causing neonatal osteoarticular infection include coagulase-negative staphylococci,28 Streptococcus pneumoniae and group A streptococcus (Table 9.1).
Candida
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


