CERVICAL CANCER
Epidemiology (CA Cancer J Clin 2011;61:212)
• 2nd most common cancer in women worldwide
• Mean age at dx: 40–59 y; bimodal distribution peaks 35–39 y & 60–64 y
• 60% women w/ cervical cancer in developed countries were never screened or were not screened in past 5 y
Pathology (J Clin Pathol 1998;51:96)
• Squamous cell carcinoma: 80% of invasive cervical cancer
• AdenoCa: 20–25% of invasive cervical cancer. In 15%, lesion not visible (located w/i endocervical canal)
Mucinous adenoCa: Most common type (well differentiated)
Endometrioid carcinoma: 30% of cervical adenocarcinomas
Clear cell carcinoma: 4% of adenocarcinomas. DES exposure ↑ risk
• Adenosquamous carcinoma: Benign & malig glandular & squamous elements. More aggressive than adenoCa.
• Small cell carcinoma: Neuroendocrine tumors. Clinically aggressive; ↑ propensity for metastases; a/w HPV18; CD56 marker often positive
Etiology (J Pathol 1999;189(1):1)
• Risk factors (Int J Cancer 2007;120:885)
Lack of cervical cancer screening. Cigarette smoking: 2–3 fold ↑ risk in current & former smokers. Multi sexual partners (more than 6 partners significantly ↑ risk). HPV infxn.
H/o STIs. Early age of sexual activity. ↑ parity. Long-term combined OCP use (higher hormone levels make cells vulnerable to mut). Immunosuppression (esp HIV). Low socioeconomic status. DES exposure in utero. No known racial predilection but mortality rate for black > white.
Role of HPV
• HPV detected in 99% of cervical cancer
• HPV types 16, 18, 31, 33, & 45 → high-risk types. Most common HPV 16 & 18.
E1–E7 (early oncoproteins in cervical cancer) expressed in HPV positive cases (E1 & E2 → viral replication; E6 & E7 → viral transformation). E6 & E7 form complexes w/ p53 & pRB (tumor suppressor genes); E6 inactivates p53; E7 inactivates Rb.
• CIN: Precursor lesion. Cervical cancer may take >10 y.
CIN 1: 57% spontaneously regress; 1% progress to carcinoma
CIN 2: 43% spontaneously regress; 5% progress to carcinoma
CIN 3: 30% spontaneously regress; 12% progress to carcinoma
Clinical Manifestations (J Clin Pathol 1998;51:96)
• Abn uterine bleeding or postcoital bleeding. Vaginal discharge (serosanguinous or yellow, foul smelling). Hematometra: Pelvic pain, difficulty w/ urination or defecation. Metastatic dz: Back pain, leg swelling (usually unilateral), & neuropathic pain.
• Exam: Firm barrel-shaped cervix; necrotic or friable lesion on cervix, poss extension into parametrium, vagina pelvic sidewall, & uterosacral ligament
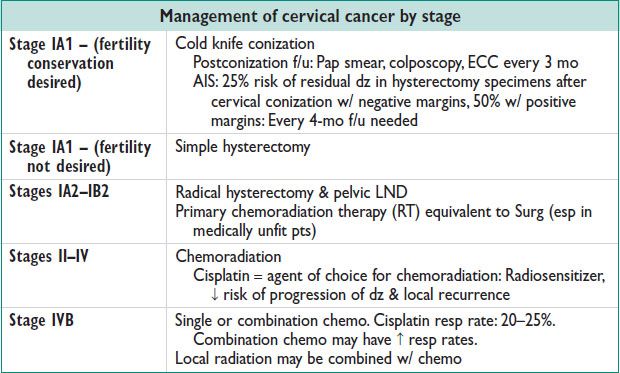
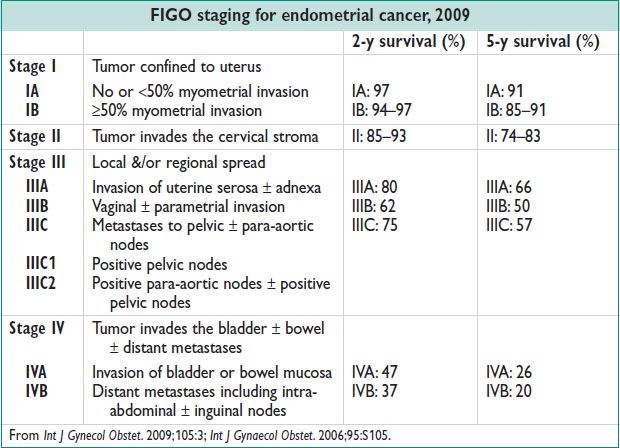
Diagnostic Workup and Staging (see Table)
• Cervical Cytology Screening Guidelines (American Society for Colposcopy and Cervical Pathology (ASCCP), American Cancer Society (ACS), and U.S. Preventive Services Task Force (USPSTF)). See Ch. 1.
• Clinically staged. Advanced imaging does not influence staging dx.
• Inspection, palpation, CXR, colposcopy, cystoscopy, proctoscopy, IVP, bx of exophytic cervical lesions; cervical conization
• Preoperative imaging may guide mgmt. PET superior to CT & MRI for imaging of nodal dz: PET sens = 84%.
Treatment (see Table) (Gynecol Oncol 1980;9:90; Gynecol Oncol 1980;32:135)
• Surg: An option for stages IIA or less
• Chemo & RT: An option for stages IA2–IVB
• Recurrent cervical carcinoma: Evaluated w/ PET scan to exclude distant metastases
Localized recurrence after Surg → RT or chemoradiation or Surg
Central recurrences after definitive Surg or adjuvant RT: Pelvic exenteration. Rpt RT considered in selected pts.
• Fertility sparing Surg:
Radical trachelectomy (pts w/ up to stage IB1; tumor size <2 cm) similar recurrence rates to radical hysterectomy in carefully selected pts.
Cervical conization in stage IA1 cancers
Posttreatment Surveillance
• Cancer detected w/i the 1st 6 mo after rx = persistent cancer
• F/u exam w/ pap q3mo × 2 y, then q6mo × 3 y, then annually
Cervical Carcinoma in Pregnancy (Best Pract Res Clin Obstet Gynaecol 2005;19:611)
• Stage IA1: Follow w/ colposcopy each trimester; surgical rx after vaginal deliv if invasion <3 mm & no LVSI. Risk of hemorrhage at deliv ↑. Or C-section + simple hysterectomy (stage IA1) if childbearing complete.
• Stage IA2 (Tumors >3–5-mm invasion): Can be followed until term; modified radical hysterectomy + pelvic lymphadenectomy at deliv or 6 w postpartum. Vaginal deliv acceptable; C-section necessary for stage IB & above.
• Stages IB1–IIA dz: Delay of rx can impact survival; if dx made after 20 w, rx can be postponed→ classical C-section; modified radical hysterectomy + pelvic/para-aortic LND. RT is as effective as Surg.
• Invasive cancer: If at or near term, immediate deliv & definitive rx is recommended. At gestational age <20 w, termination of Preg & definitive rx is an option.
• Neoadjuvant chemo in Preg may be an option for stages IB2–IIB after appropriate counseling
UTERINE CANCER
Epidemiology (Obstet Gynecol; 2005;104:65:413; J Natl Med Assoc 2006;98:1930; Cancer Control 2009;16:53)
• Most common gynecologic malig; 4th most common cancer in females
• 8th leading cause of cancer-related death among women in US
• Lifetime incid: 2.6%; White > Black > Hispanic > Asian. Mortality: Black > White.
• Median age at dx: 67 y (5% <40 y; 90% >50 y)
• Tumors confined to the uterus in 75% of cases
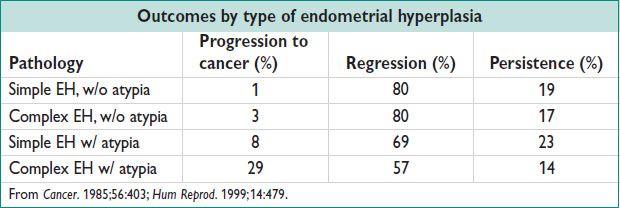
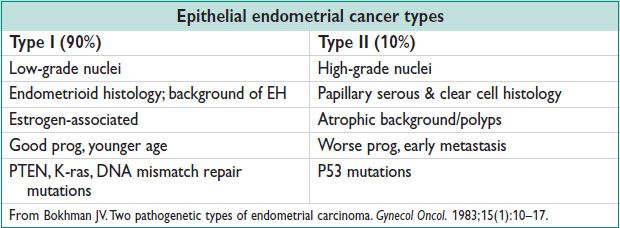
Endometrial Hyperplasia (EH) (Cancer 1985;56:403)
• Precursor lesion of endometrioid EC. From continuous estrogen stimulation & relative progestin deficiency. Classification based on architecture (simple vs. complex) & cytologic features.
Simple EH (w/o atypia): ↑ gland proliferation; abundant stroma; no nuclear atypia
Complex EH (w/o atypia): ↑ gland:stroma ratio; crowded irreg glands; no nuclear atypia
Simple EH w/ atypia: ↑ gland:stroma ratio; simple appearing glands; glands lined by atypical nuclei
Complex EH w/ atypia: Markedly ↑ gland:stroma ratio, severely crowded glands; nuclear atypia
• D&C req prior to rx to rule out occult carcinoma. 43% have EC diagnosed at the time of hysterectomy for hyperplasia (Cancer 2006;106:1012)
• Rx of EH w/o atypia:
Progestins (cyclic or continuous); eg, MDPA 10 mg/d for 12–14 d for 3–6 mo or local progestogen (LNG IUD) or OCPs. Postmenopausal women: MDPA; D&C for f/u
F/u: Rpt endometrial sampling if abn bleeding recurs
• Rx of EH w/ atypia:
Hysterectomy. For fertility preservation or poor surgical candidates: LNG IUD or continuous progestins: Megestrol acetate (40–60 mg 2–4 times/d for 6 mo) → 94% regression rate. F/u: Endometrial bx or D&C q3mo for at least 1 y; if regression does not occur, progesterone dosage should be increased or hysterectomy considered.
Pathology (J Clin Oncol 2006;24:4783; Am J Surg Pathol 1994;18:687)
• Grading: Based degree of solid components, nuclear features, & architectural pattern
Grade 1: 5% or less nonsquamous or nonmorular solid growth pattern
Grade 2: 6–50% nonsquamous or nonmorular solid growth pattern
Grade 3: >50% nonsquamous or nonmorular solid growth pattern
• Epithelial tumors
Endometrioid adenoCa: 75–80% of EC; most common
UPSC: 10% of EC; closely resembles tumors of the ovary and fallopian tube. More than 50% of pts w/ stage I UPSC have extrauterine dz. Poor prog; high risk of recurrence. EIC: Poss precursor of UPSC.
Clear cell: 3–4% of EC. Poor prog; 20–65% 5-y survival
Others: Mucinous, secretory, squamous
• Mesenchymal tumors (sarcomas): 2–5% of EC
Etiology (Obstet Gynecol 2005;104:413)
• ↑ unopposed estrogen → EH → EC
• Microsatellite instability: Germ-line mut in DNA mismatch repair genes (MLH1, MSH2, MSH6, PMS2) → Lynch II syn: 25–30% of all EC; 40–60% lifetime risk of EC
• Risk factors for EC: Prolonged unopposed estrogen (RR 10); chronic anovulation (eg, PCOS); BMI >30 (RR 2–5); diabetes & HTN (independent risk factors); Tamoxifen (RR 3–7); older age (RR 2–3); nulliparity (RR 3); early menarche, late menopause (1.5–3)
• Protective factors for EC: Smoking (RR 0.5); OCPs: ↓ EC risk by 40% up to 15 y after discontinuation; 12 y of use ↓ risk by 72%
Clinical Manifestations and Physical Exam (Obstet Gynecol 2005;106:413)
• Presentation: Abn uterine bleeding (10% postmenopausal bleeding is EC); chronic anovulation; abn pap smear 30–50%; asymptomatic 5%; leukorrhea 10%; hematometra due to cervical stenosis
• Ddx: Atropic vaginitis, fibroids, endometrial polyps, cervical carcinoma, CIN
Diagnostic Workup
• Office endometrial sampling: Least invasive approach
• Pelvic US (not diagnostic but may help triage pts): ET <5 mm = 99% NPV (NEJM 1997;227:1792)
• Fractional D&C: Office endometrial bx results correlate well w/ uterine curettage & ET up to 6 mm (Acta Obst Gynecol Scand 2001;80:959)
• Cervical conization if cervical involvement suspected to rule out primary cervical carcinoma
• CA125: Elevated in women w/ advanced stage dz & UPSC. Not routinely performed.
• Chest radiograph, CT/MRI: If extrauterine dz suspected or CA125 elevated.
Management (Obstet Gynecol 2005;106:413; Int J Gynaecol Obstet 2000;70:209)
• Surg depends on stage:
All stages: Hysterectomy & BSO (std rx)
All stages: LND (pelvic & para-aortic) & staging→ allows assessment of the extent of dz to tailor adjuvant therapy. Therapeutic value in stage I dz is unk (Obstet Gynecol 2012;120:383)
Stage II → radical hysterectomy & lymphadenectomy + adjuvant therapy based on pathology
Stages III–IV → optimal cytoreductive Surg
Laparoscopic or robotic Surg not inferior to open Surg
• Radiation therapy (RT):
PORTEC trial → pelvic radiation decreases local recurrence (4.2% vs. 13.7%) but overall survival unchanged
Vaginal brachytherapy → for risk for recurrence or pts who have vaginal recurrence → 60–75% survival
Whole pelvic (external beam) adjuvant radiation may prevent vaginal/local recurrence
In poor surgical candidates, primary RT may be considered
Survival rate for pts treated w/ primary RT w/o Surg: 50% at 5 y
• Adjuvant chemo:
Rx of choice in pts w/ metastatic or recurrent endometrial cancer
Combination chemo (carboplatin & paclitaxel) → improved resp rate
Serous & clear cell cancers: Carboplatin & paclitaxel = resp rate 60–70%
• Hormonal therapy:
Occ used in rx of stage I, grade 1 dz in women who wish to maintain fertility or in poor surgical candidates; resp rates 58–100%
In pts w/ recurrent dz, overall resp rate 25%
Regular histologic (eg, endometrial bx) monitoring necessary
Posttreatment Surveillance
• Exam q3–6mo × 2 y, then q6mo for 3 y, then annually. If CA-125 elevated at the time of dx, it can be followed at each visit. Most recurrences diagnosed w/i the 1st 2 y; 10% recur >5 y after original dx. Routine chest radiographs or pap smears do NOT improve survival or outcome.
Uterine Sarcomas (Pathology 2007;39:55; Oncol 1993;50:105)
• Uncommon, arise from mesenchymal (stromal) component of uterus
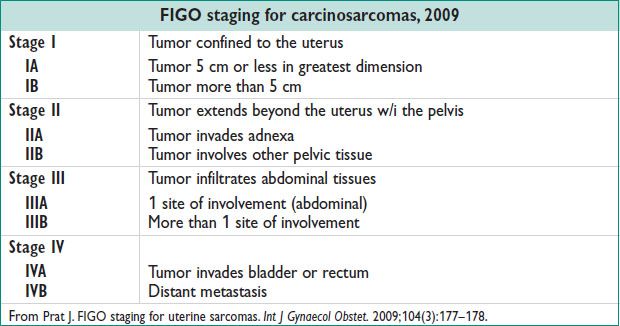
Carcinosarcoma (previously called MMMT)
Present w/ postmenopausal bleeding; median age 65 y; h/o exposure to radiation; more common in AA women; lymphatic route of spread; ↑ potential for extrauterine metastasis
Adenosarcoma:
Variable in size. Locally invasive
Endometrial stromal sarcoma
Abn uterine bleeding or asymptomatic uterine enlargement. Indolent course, may recur late. 70% are stage I or stage II at dx.
Leiomyosarcoma
Median age at dx: 55 y. Menorrhagia & pelvic mass. Hematogenous route of spread. Primary sites of recurrence: Lung (41%), pelvis (13%).
• Rx of carcinosarcoma:
Surg – hysterectomy, BSO, removal of metastatic dz
LND preferred in carcinosarcomas, controversial in leiomyosarcomas & other sarcomas
Adjuvant chemo & RT recommended
EPITHELIAL OVARIAN CANCER (EOC)
Definitions and Epidemiology (http://seer.cancer.gov/csr/1975_2008, accessed December 1, 2012)
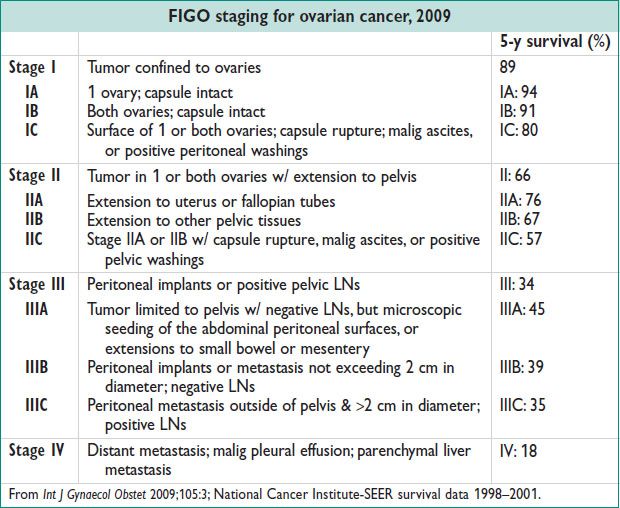
• EOC is derived from surface epithelium of ovary. Incid: 12.8/100000 women/y
• 5th leading cause of cancer death in US. 90% of all ovarian cancers.
• Lifetime risk: 1.5%. Risk of death: 1%.
• Presentation red flag sx: Incidental abdominal pain, abdominal distension, loss of appetite, rectal bleeding, postmenopausal bleeding, weight loss
Pathology (Human Pathology 2009;40:1213)
• Serous tumors: Low & high grade
• 40–50% of EOC; most common type of EOC. 60% bilateral. Psammoma bodies seen in low-grade tumors. Most common in BRCA carriers & in pts w/ Lynch syn
• Mucinous tumors (Int J Gynecol Cancer 2008;18:209)
• 10% of EOC. 8–10% bilateral
• Endometrioid adenoCa
• 10% of all ovarian cancers. 28% bilateral. 42% a/w endometriosis; 15–20% a/w endometrial carcinoma
• Clear cell cystadenocarcinoma
• 10% of all ovarian cancers. 40% bilateral. A/w endometriosis & HyperCa.
• Brenner/transitional cell carcinoma
• Rare, poorly differentiated similar to high-grade transitional cell carcinoma of bladder
• Carcinosarcoma
• 1–4% of all ovarian neoplasms. Carcinomatous & sarcomatous elements. Often stage III or stage IV at dx. Poor overall survival.
• Metastatic tumors
• Krukenberg tumor: Signet ring cell, GI tumor. Colonic adenoCa. Pancr adenoCa. Breast cancer: Accounts for 6–40% of metastatic tumors to ovary; often bilateral. Renal cell carcinoma. Burkitt’s lymphoma. Low malig potential (borderline) tumor: Mucinous or serous.
Etiology (Gynecol Oncol 2010;119:7)
• Risk factors: Nulliparity, FHx, early menarche, late menopause, white race, increasing age, residence in North America or Northern Europe, personal h/o breast cancer, European Jewish, Icelandic or Hispanic ethnicity, talc exposure
• Protective factors: Long-term OCP use, tubal ligation, hysterectomy, breastfeeding
• Hypothesis of etiology: Incessant ovulation, gonadotropin/hormone/inflammation stimulation
• Hereditary breast & ovarian cancer (& see Ch. 1, screening)
• 10% of all ovarian cancers. BRCA1, BRCA2, & Lynch syn. Autosomal dominant.
• Lifetime risk w/ mut: 28–44%; higher w/ BRCA1. Cancer occurs 10 y earlier.
Diagnostic Workup
• Pelvic US: Complex adnexal mass (septations &/or solid components, size, wall loculation, papillary projections)
• Abdominopelvic CT or MRI: Complex adnexal mass, omental caking, ascites, peritoneal studding, perihepatic diaphragmatic implants, CA-125 ↑ esp w/ serous tumors
• Refer to gynecologic oncologist if complex adnexal mass, elevated CA-125, ascites, significantly elevated CA-125 in premenopausal (>200 U/mL) or postmenopausal (>35 U/mL) women, FHx of breast or ovarian cancer in 1st-degree relative (Obstet Gynecol 2007;110:201)
Management
• Preventative. BRCA1/BRCA2 carriers: Risk reducing BSO by age 40 or completion of child bearing
• Surgery
Stage I: TAH, BSO, omentectomy, peritoneal biopsies, pelvic & para-aortic LND, pelvic washings
Stage I w/ desired fertility: Fertility sparing Surg w/ unilateral salpingo-
oophorectomy, peritoneal biopsies, omentectomy, pelvic & para-aortic LND, pelvic washings
Stages II–IV: TAH, BSO, omentectomy, debulking of gross dz; optimal reduction to residual dz <1 cm
• Adjuvant chemo: Grade III or stage IC or higher: Postsurgical systemic chemo w/ platinum & paclitaxel.
• Neoadjuvant chemo: Used for pts who are not initial surgical candidates. Adjuvant RT not recommended.
• Recurrent/persistent dz (Clin Obstet Gynecol 2012;55:114)
Carboplatin + paclitaxel for platinum sensitive dz (recurrence >6 mo from rx). Single-agent rx w/ alternative chemo agent (eg, topotecan, paclitaxel, docetaxel, gemcitabine) for platinum resistant (recurrence <6 mo from rx) or platinum refrac dz (progression during rx).
• Carcinosarcoma/MMMT: Surg + platinum-based chemo + paclitaxel or ifosfamide; role of radiation unk.
Posttreatment Surveillance (Am J Obstet Gynecol 2011;204:466)
• Exam ± CA-125 q3mo for 3 y, then q6mo for 2 y, then yearly
• CT &/or PET, CA-125 if recurrence suspected
GERM CELL TUMORS
Definitions and Epidemiology (Cancer Treat Rev 2008;34:427)
• Cancer derived from primordial germ cells. 1–2% of all ovarian malignancies
• 58% of all ovarian tumors in women <20 yo. Incid: 0.41/100000 women/y
Pathology (Int J Gynecol Path 2006;25:305)
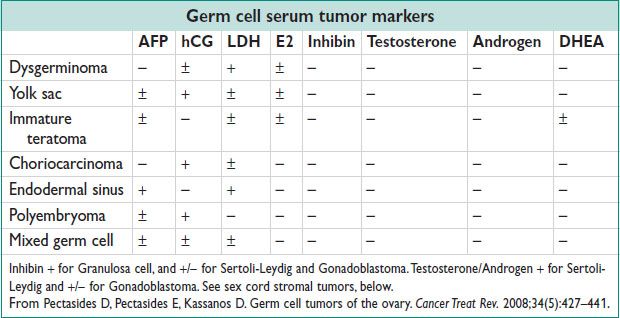
• Dysgerminomas
1–2% all of ovarian tumors; 32% of malig germ cell tumors. Adolescents/young adults.
10–15% bilateral. Monophasic proliferation of primitive germ cells w/ infiltrating T cells.
Testicular seminoma equivalent; OCT4 positive & CD30 positive staining
Lymphatic spread common; humoral HyperCa common; rapid enlargement
High cure rate w/ rx (88.6%)
• Endodermal sinus tumor (yolk sac tumor)
14–20% of malig germ cell tumors. Young girls/young women; 1/3 premenarchal
Schiller–Duval bodies (microscopic feature w/ central capillary surrounded by flattened parietal cells). AFP, cytokeratin, & PLAP positive staining.
Unilateral, aggressive tumor
• Embryonal carcinoma
4% of malig germ cell tumors. Avg age: 15 yo.
Cohesive groups of large primitive cells w/ overlapping nuclei, indistinct borders, syncytiotrophoblastic giant cells. hCG production leads to isosexual pseudoprecocity. Staining positive for OCT3, OCT4, & CD30.
• Polyembryoma
Young girls. Numerous embryoid bodies resembling presomite embryos. hCG/AFP may be elevated.
• Nongestational choriocarcinoma
2% of malig germ cell tumors. Cytotrophoblasts & intermediate trophoblasts capped w/ syncytiotrophoblasts in plexiform pattern. hCG, hPL, inhibin, & cytokeratin positive.
Early hematogenous spread to distant sites. Relatively chemoresistant.
• Mixed germ cell tumor
5% of all malig germ cell tumors. 2 or more malig germ cell elements w/ at least 1 primitive. Dysgerminoma most common component.
• Immature teratoma
Embryonic tissue; predominantly neuroepithelial. Grade 1, 2, or 3 based on quantity of neuroepithelial tissue. Unilateral.
• Mature teratoma
Solid, cystic (dermoid, 95%), or fetiform. Composed of fetal or adult structures, no embryonal components. Most common ovarian tumor. 46XX karyotype.
Only 1–2% malig; most common malignancy is squamous cell carcinoma.
• Monodermal teratomas
Struma ovarii, carcinoid, central nervous center tumor, carcinoma group, sarcoma group, sebaceous tumor, pituitary-type tumor, retinal anlage tumor, others.
Clinical Manifestations
• Abdominal pain (55–80%), abdominal/pelvic mass, abdominal enlargement, fever (10–25%), ascites, ovarian torsion or rupture; abdominal distension (35%), vaginal bleeding (10%)
• Short duration of sx (2–4 w)
• 60–70% present at stage I or stage II, 20–30% stage III, stage IV uncommon. Metastasis by peritoneal or lymphatic spread; hematogenous spread more common than EOC.
• Dysgerminoma a/w primary amenorrhea/gonadal dysgenesis
Diagnostic Workup
• Chest radiograph: Eval for metastasis
• Pelvic US: Cystic lesion w/ densely echogenic tubercle (Rokitansky nodule for mature teratoma). CA-125 not useful.
• Abdominal/pelvic CT: Complex mass; fat attenuation in mature teratomas; calcification; speckled calcification in dysgerminomas (Radiographics 1998;18:1525)
• Karyotype if dysgerminoma suspected & h/o primary amenorrhea
• Staging same as for EOSs, above.
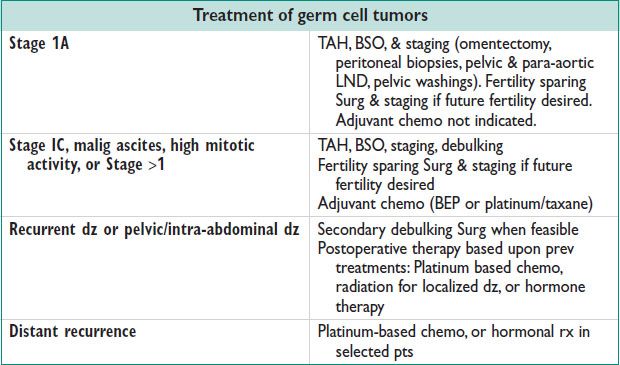
Management
• Surg: TAH, BSO, omentectomy, peritoneal biopsies, pelvic washings, pelvic & para-aortic LND, surgical debulking if not sparing fertility.
Fertility sparing Surg poss if contralateral ovary appears nml; cystectomy may be poss. Bx contralateral ovary if dysgerminoma or if appears abn.
Second-look Surg if residual mass postchemotherapy or residual teratoma
• Adjuvant chemo:
BEP (bleomycin, etoposide, cisplatin) is gold std
Recurrence treated w/ chemo again
• Primary surveillance: Option for stage IA or IB
• Adjuvant radiation (RT): Alternative therapy for dysgerminomas
Posttreatment Surveillance (Am J Obstet Gynecol 2011;204:466)
• Exam & tumor marker(s) q2–4mo for 2 y, then yearly. Imaging w/ surveillance if no reliable tumor marker. CT & tumor marker(s) if recurrence suspected
• Overall prog based on stage, residual dz, histologic type, preop AFP & bhCG elevation; age not a factor
SEX CORD-STROMAL TUMORS
Epidemiology (J Clin Oncol 2007;25:294)
• 7% of all malig ovarian neoplasms. Indolent course w/ favorable prog.
Pathology (J Clin Oncol 2007;25:294)
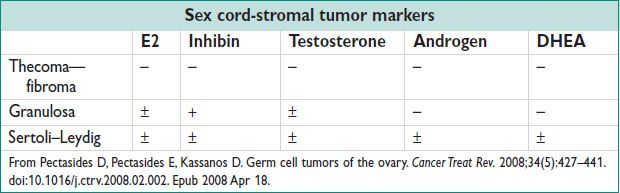
• Granulosa cell tumor (GCT):
70% of malig sex cord-stromal tumors. Incid: 0.4–1.7/100000 women. More common in nonwhite, obese women.
3–5% of all ovarian neoplasms. Adult type-estrogen production w/ abn bleeding in 66%; EH 25–50%; endometrial cancer 5%.
Juvenile type: 90% in prepubertal girls; 95% unilateral; excellent prog.
Call–Exner bodies w/ eosinophilic material & nuclear debris, coffee bean nuclei. 95% unilateral. 78–91% stage I at dx; good prog.
• Sertoli–Leydig cell tumors:
0.2% of all ovarian neoplasms. 98% unilateral. Avg age 20–30 y.
90% stage I; 70–90% 5-y survival; may recur soon after dx/rx
Tubules of epithelial cells are steroid secreting
• Thecoma:
Benign. Postmenopausal women. Estrogen → EH (15%).
Luteinized thecomas → virilization. Abundant lipid cytoplasm; solid, yellowish tumors.
• Fibroma:
Benign. Most common sex cord-stromal tumor; 4% ovarian neoplasms.
4–8% bilateral. Postmenopausal women. Whorled bundles of spindle-shaped fibroblasts & collagen. A/w Meigs syn & basal nevus syn.
• Steroid cell tumors: 0.1–0.2% of all ovarian tumors. Stromal luteomas, Leydig (hilus) cell tumor, & steroid cell tumor not otherwise specified.
• Others: Sclerosing stromal tumors, sex cord tumor w/ annular tubules, gynandroblastomas
Clinical Manifestations
• Presentation: Abn bleeding, abdominal distension, abdominal pain. Isosexual precocious puberty w/ juvenile GCTs. Virilization from androgens in Sertoli–Leydig. Meigs syn (fibroma, ascites, pleural effusions).
Diagnostic Workup (Radiographics 1998;18:1525)
• Pelvic US/Pelvic CT: Large, unilateral, multicystic w/ solid components; rare calcifications; carcinomatosis in GCTs (rare); well-defined hypoechoic mass for Sertoli–Leydig cell tumors; lack of papillary projections
• Pelvic MRI: High signal intensity due to tumor hemorrhage; GCTs w/ sponge-like appearance; Sertoli–Leydig cell tumors as solid mass; fibrothecomas w/ low signal intensity on T2
• Staging same as for EOSs, above
Management
• Surg: TAH, BSO, omentectomy, peritoneal biopsies, pelvic & para-aortic LND, pelvic washings. Fertility sparing Surg when poss & desired. Endometrial sampling w/ granulosa cell tumors, for hyperplasia. See tables.
Posttreatment Surveillance (Am J Obstet Gynecol 2011;204:466)
• Exam & tumor marker(s) q2–4mo for 2 y, then q6mo for 3 y, then yearly
• CT & tumor marker(s) if recurrence suspected
VAGINAL CANCER
Epidemiology
• 1–2% of all gynecologic malignancies. Incid of VAIN: 0.2/100000 women
• Mean age: 70–90 y. 84% are metastases from other sites.
Pathology (Curr Opin Obstet Gynecol 2005;17:71)
• VAIN is precursor lesion. Upper 3rd of vagina most common. A/w CIN. Risk of transformation to invasive vaginal carcinoma 9–10%.
• Squamous cell carcinoma
85% of vaginal cancer. Superficial spread, then invasion to paravaginal tissue. Metastasis to liver/lung.
• AdenoCa:
15% of cases. Metastasis to lung, supraclavicular & pelvic LNs. Metastasis from other sites is more common than primary vaginal adenoCa.
• Clear cell adenoCa: DES exposure. Coexists w/ vaginal adenosis.
• Melanoma: <1–3% of vaginal malignancies. Pigmented or nonpigmented.
• Sarcoma botryoides: Multicentric; anter wall; grape like. More common in children.
• Adenosquamous carcinoma: 1–2% of vaginal cancer. Aggressive.
• Secondary carcinomas: Extension from cervix, endometrial metastasis, bowel/bladder local extension, gestational trophoblastic dz.
Etiology
• HPV 16 & 18 found in invasive cancer & VAIN. DES exposure. Endometriosis linked w/ adenoCa. Radiation exposure.
Clinical Manifestations
• Vaginal bleeding or bloody discharge usually indicates advanced lesions. Urinary sx.
Diagnostic Workup
• Bx for tissue dx; view by colposcopy w/ Lugol’s solution (localized or skip lesions). Bx cervix & vulva as well.
Management
• VAIN I: Observation
• VAIN II or III: Wide local excision, partial or total vaginectomy, intravag 5-FU, trichloroacetic acid, 5% imiquimod, laser therapy (Journal of Lower Genital Tract Disease 2012;16:00)
• Stage I SCC: <0.5 cm thick: Intracavitary radiation, wide local excision, or total vaginectomy; >0.5 cm thick: Radical vaginectomy w/ pelvic LND & inguinal LND (if lower 3rd), radiation if lower 3rd to pelvic/inguinal LNs or poorly differentiated/infiltrating.
• Stage I adenoCa: Total radical vaginectomy, hysterectomy, LND, vaginal reconstruction ± intracavitary/interstitial radiation
• Stage II SCC/adenoCa: Brachytherapy/EBRT or radical vaginectomy or pelvic exenteration ± radiation
• Stages III & IVA SCC/adenoCa: Interstitial, intracavitary, & EBRT
• Stage IVB SCC/adenoCa: Radiation ± chemo
• Melanoma: Wide local excision, radical excision w/ inguinofemoral LND, pelvic exenteration, radiation, chemo, or immunotherapy (Int J Gynecol Cancer 2004;14:687)
• Local recurrence: Pelvic exenteration or radiation
• Distant recurrence: Chemo
• Prog: 70% 5-y survival for stage I; 50% survival for advanced stage

Posttreatment Surveillance (Am J Obstet Gynecol 2011;204:466)
• Exam (if low risk) q6mo × 2 y then yearly × 2 y; (if high risk) q3mo × 2 y, then q6mo × 2 y, then yearly. Pap smear yearly. CT or PET if recurrence.
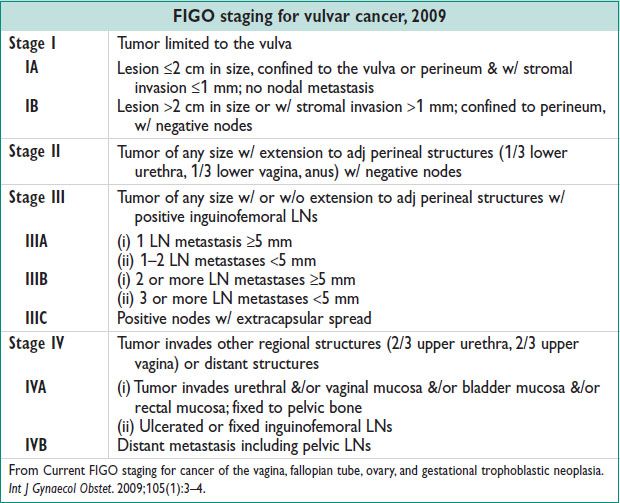
VULVAR CANCER
Definitions and Epidemiology (Hematol Oncol Clin N Am 2012;26:45)
• VIN: Dysplasia confined to epithelium
• Vulvar carcinoma: Lesion invading through basement membrane
• Incid: Vulvar cancer 2.3/100000 women/y; VIN: 1.2–2.1/100000 women
• 4–7% of all gynecologic malignancies. Median age at dx: 68 y.
• Lifetime risk: 0.27%
Pathology
• VIN usual type: Warty, basaloid, mixed. HPV related.
• VIN differentiated type: A/w lichen sclerosus, squamous cell hyperplasia. NOT HPV related. Risk of developing keratinizing squamous cell carcinoma.
• SCC: 92% of vulvar cancer. Warty & basaloid type; keratinizing, nonkeratinizing, basaloid, verrucous, warty, & acantholytic type; invasive or superficial invasion. Most common sites: Labia majora (50%), labia minora (15–20%). HPV16 & 18; 40% of invasive cancers are HPV positive; 80% of VIN are HPV positive; vaccination may prevent.
• Basal cell carcinoma: 2–4% of vulvar malignancies. Infiltrating tumor w/ basal cells of the epidermis. Labia majora is the most common site. Basosquamous or metatypical basal cell carcinoma: Malig squamous component, found in 3–5% of basal cell carcinomas (treat as squamous carcinoma).
• Bartholin’s gland carcinoma: 40% adenoCa; 40% squamous carcinoma; 15% adenoid cystic carcinoma. Bx any Bartholin’s gland abscess in woman >35 y.
• Sarcoma: 1–2% vulvar malignancies. Leiomyosarcoma, liposarcoma, fibrosarcoma, neurofibrosarcoma, rhabdomyosarcoma, malig schwannoma, angiosarcoma, epithelioid sarcoma.
• Verrucous carcinoma: Rare. Cauliflower-like appearance. Slow growing & locally invasive (will even invade bone)
• Malig melanoma: 2nd most common vulvar malig. Labia minora or clitoris most common sites. Arise de novo; pigmented lesion, asymptomatic.
• Paget’s dz of vulva: <1% of vulvar neoplasms. Concurrent w/ underlying adenoCa in 4–20%. 12% invasive; 35% recurrence rate. Large pale cells (Paget cells). Raised, velvety appearance. A/w adenoCa of other location (breast/colon): 30%.
Clinical Manifestations
• Presentation: Vulvar itching & irritation, burning, pain, dysuria. Pigmented lesions, ulcerations, papules, nodules, or scar-like lesions. Persistent condyloma (30% w/ VIN 3).
Diagnostic Workup
• Bx flat, elevated, or pigmented lesions; bx genital warts in postmenopausal women or women who fail topical therapy. Colposcopy.
Management
• VIN: Wide local excision (low risk of recurrence if negative margins); laser ablation if cancer not suspected (colposcopy to delineate margins); topical 5% imiquimod
• Vulvar squamous carcinoma
Stage I: Wide local excision if microinvasive (<1 mm invasion), otherwise, radical local excision w/ complete unilateral LND (bilateral LND if lesion <1 cm from midline)
Stage II: Modified radical vulvectomy w/ bilateral inguinal LND & femoral LND: Radiation if margins <8 mm, lymphovascular invasion, or >5 mm thick
Stage III: Modified radical vulvectomy w/ bilateral inguinal/femoral LND w/ radiation
Stage IV: Radical vulvectomy followed by radiation
Recurrence: Depending on location & extent of recurrence, options include wide local excision, radical vulvectomy, pelvic exenteration, radiation, chemo
• Basal cell carcinoma: Radical local excision
• Bartholin’s gland carcinoma: Radical local excision or hemivulvectomy, consider ipsilateral inguinal LND
• Sarcoma: Radical local excision
• Verrucous carcinoma: Radical local excision; radiation contraindicated (induces anaplastic transformation which may lead to metastasis)
• Malig melanoma: Radical local excision if <1 mm invasion; consider ipsilateral inguinal LND if >1 mm invasion
• Paget’s dz of vulva: Wide local excision; modified radical vulvectomy if underlying adenoCa
• Prog: 5-y survival 72.7%; based on stage at dx; ↑ risk of metastasis if nodes positive, advanced stage, advanced age, increased stromal invasion, LVSI
Posttreatment Surveillance (Am J Obstet Gynecol 2011;204:466)
• Exam q3mo × 2 y, then q6mo × 3 y, then yearly.
CT &/or PET if recurrence suspected. VIN surveillance: q6mo for 1 y, then annually; recurrence high (30–50%).
GESTATIONAL TROPHOBLASTIC NEOPLASIA
Definition and Epidemiology
• Originates from abn proliferation of placental trophoblasts. Incid varies by geography (2/1000 in Japan, 0.6–1.1/1000 in Europe/North America) (NEJM 1996;335:1740)
• GTN includes 4 types of related tumors: Complete & partial hydatidiform mole, invasive mole, placental site trophoblastic tumor, & choriocarcinoma. Invasive GTN usually follows molar Preg, but can follow any gest.
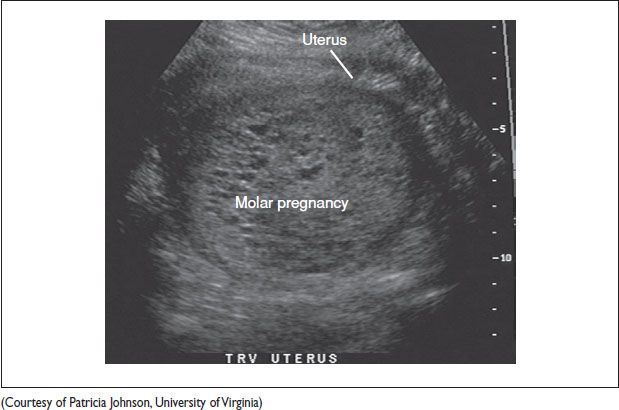
Molar Pregnancy
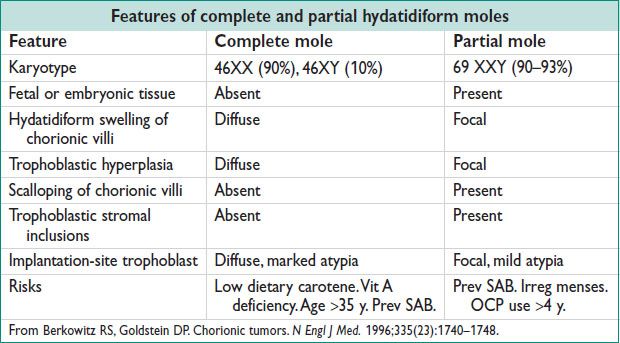
• Clinical presentation (NEJM 1996;335:1740)
Complete hydatidiform mole: Vaginal bleeding (89–97%); enlarged uterus for gestational age (38–51%); Theca lutein ovarian cysts (26–46%); hyperemesis gravidarum (20–26%); preeclampsia (12–27%); hyperthryoidism; respiratory distress (2–27%)
Partial hydatidiform mole: Signs & sx of incomplete or missed abortion; SGA or IUGR; less likely to present w/ medical complications
Diagnostic w/u pelvic US, serum hCG level, CBC, PT/PTT, renal & liver fxn studies, type & screen, pre-evacuation chest radiograph, if exhibiting sx of hyperthyroidism → TSH, T3/T4; hyperemesis → chemistry
Figure 21.1 Transverse uterus ultrasound image of a molar pregnancy with characteristic snowstorm pattern