Definitions
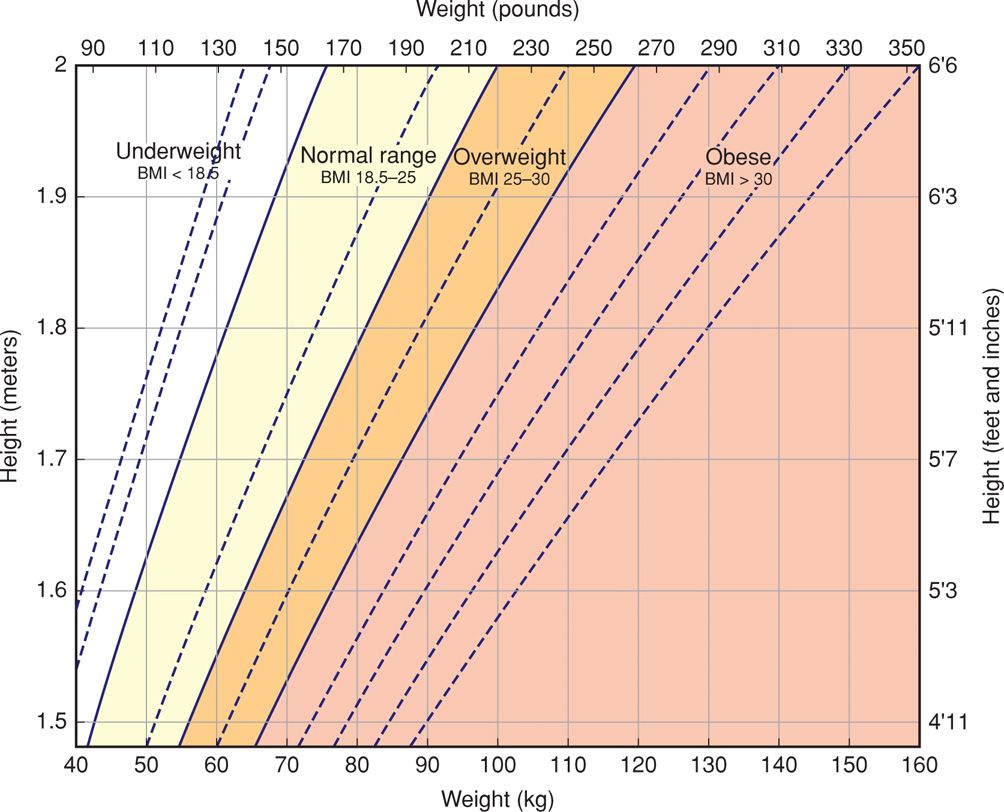
A number of systems have been used to define and classify obesity. The body mass index (BMI), also known as the Quetelet index, is currently most often used. The BMI is calculated as weight in kilograms divided by the square of the height in meters (kg/m2). Calculated BMI values are available in various chart and graphic forms, such as the one shown in Figure 48-1. The National Institutes of Health (2000) classifies adults according to BMI as follows: normal (18.5 to 24.9 kg/m2); overweight (25 to 29.9 kg/m2); and obese (≥ 30 kg/m2). Obesity is further divided into: class 1 (30 to 34.9 kg/m2); class 2 (35 to 39.9 kg/m2); and class 3 (≥ 40 kg/m2).
FIGURE 48-1 Chart for estimating body mass index (BMI). To find the BMI category for a particular subject, locate the point at which the height and weight intersect.
 Prevalence
Prevalence
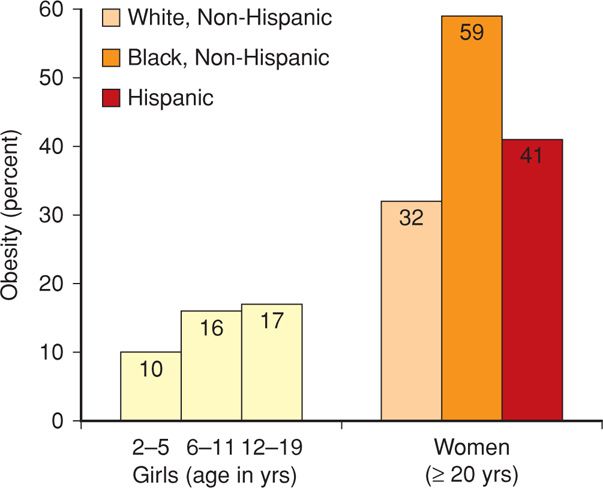
By 2000, 28 percent of men and 33 percent of women were obese (Ogden, 2012). For the period 2009 to 2010, among men and women these percentages were almost identical at approximately 35 percent. Shown in Figure 48-2 are the prevalences of obesity among girls and women. Obesity increases with age as well as with ethnic minority, and almost 60 percent of black women were obese in 2010. This is also true among indigent individuals (Drewnowski, 2004).
FIGURE 48-2 Prevalence of obesity in girls and women in the United States for 2009–2010. (Data from Flegal, 2012; Ogden, 2012.)
 Adipose Tissue as an Organ System
Adipose Tissue as an Organ System
Fat tissue is much more complex than its energy storage function. Many cell types in fat tissue communicate with all other tissues via endocrine and paracrine factors—adipokines, or adipocytokines. Some of those with metabolic functions include adiponectin, leptin, tumor necrosis factor-α (TNF-α), interleukin 6 (IL-6), resistin, visfatin, apelin, vascular endothelium growth factor (VEGF), lipoprotein lipase, and insulin-like growth factor (Briana, 2009; Scherer, 2006). A principal adipokine is adiponectin, which is a 30-kDa protein. It enhances insulin sensitivity, blocks hepatic release of glucose, and has cardioprotective effects on circulating plasma lipids. An adiponectin deficit leads to diabetes, hypertension, endothelial cell activation, and cardiovascular disease.
Adipocytokines in Pregnancy
Cytokines that result in insulin resistance—leptin, resistin, TNF-α, and IL-6—are increased during pregnancy. Indeed, these may be the primary stimulant of insulin resistance. Secretion of the remaining adipokines is either unchanged or decreased. Specific patterns have been variously described with gestational diabetes, preeclampsia, and fetal-growth restriction (Briana, 2009). In a longitudinal study of 55 pregnant women, Meyer and associates (2013) confirmed that higher BMIs are associated with lower adiponectin but higher leptin levels.
 Metabolic Syndrome
Metabolic Syndrome
Given its multifaceted endocrine and paracrine functions, it is not surprising that excessive fat tissue is detrimental (Cornier, 2011). One major drawback is that obesity interacts with inherited factors to cause insulin resistance and in some cases, the metabolic syndrome. This resistance is characterized by impaired glucose metabolism and a predisposition to type 2 diabetes. Insulin resistance also causes several subclinical abnormalities that predispose to cardiovascular disease and accelerate its onset. The most important among these are type 2 diabetes, dyslipidemia, and hypertension, which define the metabolic syndrome.
Criteria used by the National Institutes of Health (2001) to define the metabolic syndrome are shown in Table 48-1. Virtually all obese women with hypertension demonstrate elevated plasma insulin levels. These are even higher in women with excessive fat in the abdomen—an apple shape—compared with those whose fat is in the hips and thighs—a pear shape (American College of Obstetricians and Gynecologists, 2003).
TABLE 48-1. Criteria for Diagnosis of the Metabolic Syndrome
Patients with three or more of the following:
Waist circumference: > 88 cm (34.7 in) in women; > 102 cm (40.2 in) in men
Hypertriglyceridemia: ≥ 150 mg/dL
High-density lipoprotein (HDL): < 50 mg/dL in women; < 40 mg/dL in men
High blood pressure: ≥ 130/85 mm Hga
High fasting glucose: ≥ 110 mg/dLa
Prevalence
Ford and colleagues (2002) did a follow-up study of men and women enrolled in the Third National Health and Nutrition Survey (NHANES III). They found an overall prevalence of the metabolic syndrome in 24 percent of women and 22 percent of men. As expected, prevalence increased with age. For women, prevalence was approximately 6 percent in those 20 to 29 years; 14 percent in those 30 to 39 years; 20 percent in those 40 to 49 years; and 30 percent for women older than 50 years. Jordan and colleagues (2012) reported similar figures in adults in New York City in 2004.
 Nonalcoholic Fatty Liver Disease (NAFLD)
Nonalcoholic Fatty Liver Disease (NAFLD)
Generally speaking, visceral adiposity correlates with hepatic fat content (Cornier, 2011). With obesity, excessive fat accumulates in the liver—hepatic steatosis. Specifically, in persons with the metabolic syndrome, steatosis can progress to nonalcoholic steatohepatitis (NASH) and cirrhosis. Indeed, a fourth of cases of chronic liver disease in Western countries are caused by nonalcoholic fatty liver disease (NAFLD) (Targher, 2010). Moreover, NAFLD either is a marker for cardiovascular disease or is involved in its pathogenesis. This may be related to an inherited prothrombotic state (Verrijken, 2014).
Pregnancy
Experience with NAFLD in pregnancy is limited (Page, 2011). It also appears that the increased insulin resistance it imposes causes excess gestational diabetes (Forbes, 2011). In addition, insulin resistance mobilizes free fatty acids to increase their plasma levels. In nonpregnant adults, hepatic fat content normally is 1 to 5 percent of liver mass, however, this has not been studied in pregnant women (Browning, 2004). However, Meyer and associates (2013) found that overweight and obese gravidas had a higher proportion of low-density lipoprotein III (LDL-III) compared with that of normal-weight women. LDL-III predominance is a hallmark of ectopic liver fat accumulation that is typical of NAFLD. At Parkland Hospital, with increasing frequency we are encountering obese women who have NAFLD with evidence of steatohepatitis manifest by elevated serum hepatic aminotransferase levels. In some cases, liver biopsy is necessary to exclude other causes.
MORBIDITY AND MORTALITY ASSOCIATED WITH OBESITY
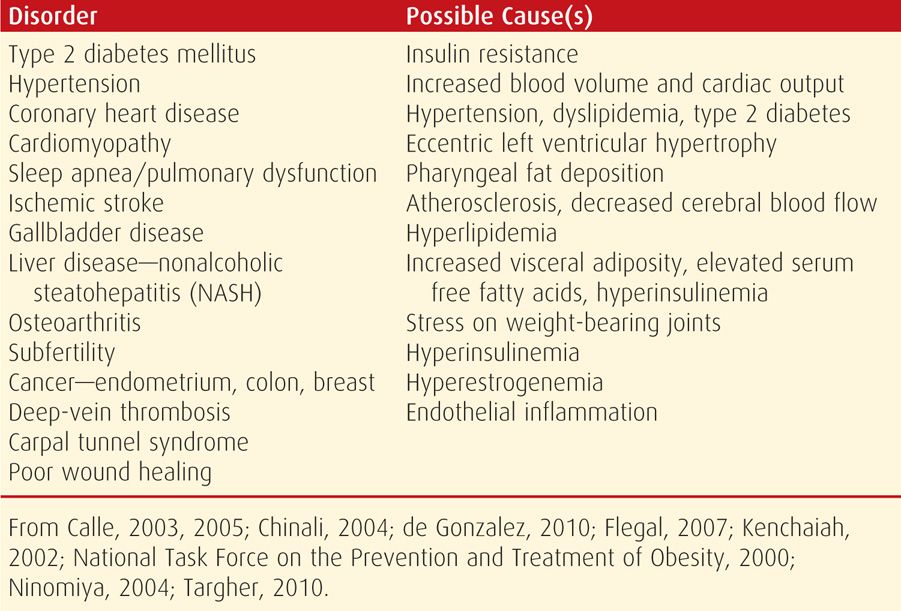
Obese individuals are at increased risk for an imposing number of complications (Table 48-2). The direct link between obesity and type 2 diabetes mellitus is well known. Ninety percent of type 2 diabetes cases are attributable to excess weight, and 75 percent of these diabetics have the metabolic syndrome (Hossain, 2007). Heart disease due to obesity—adipositas cordis—is caused by hypertension, hypervolemia, and dyslipidemia. Higher rates of abnormal left ventricular function, heart failure, myocardial infarction, and stroke have been noted (Chinali, 2004; Kenchaiah, 2002; Targher, 2010).
TABLE 48-2. Long-Term Complications of Obesity
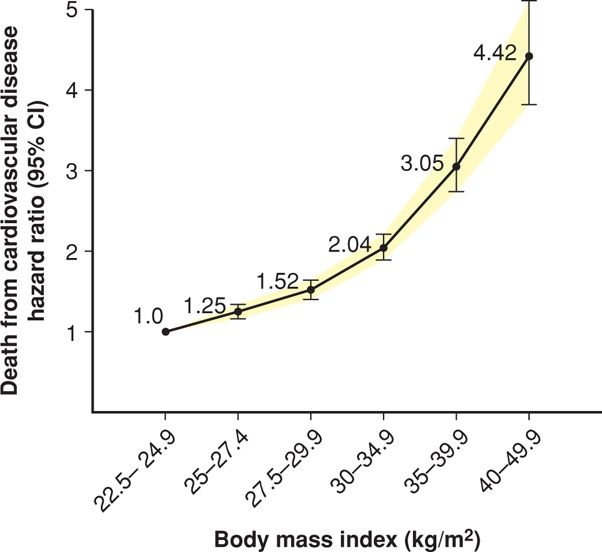
Excessive weight is associated with increased rates of early mortality, as shown by Peeters (2003) and Fontaine (2003) and their colleagues in follow-up studies from both the Framingham Heart Study and the NHANES III cohort. Mortality results from pooled data from 19 prospective studies are shown in Figure 48-3. In these and other studies, mortality risk from cardiovascular disease and cancer increased directly with increasing BMI.
FIGURE 48-3 Estimated hazard ratios (95% CI) for death due to cardiovascular disease according to body mass index among 1.46 million white adult men and women. (Data from de Gonzalez, 2010.)
TREATMENT OF OBESITY
Weight loss is tremendously difficult for obese individuals. If achieved, long-term maintenance poses equivalent or even more daunting difficulties. Even the most legitimate nonsurgical methods are fraught with frequent failure. If they are successful, slow and inexorable return to preintervention weight usually follows (Yanovski, 2005). Successful weight loss approaches include behavioral, pharmacological, and surgical techniques or a combination of these methods (Eckel, 2008; Zimmet, 2012). As such, obstetrician-gynecologists are encouraged to aid assessment and management of obesity in adult women. Weight loss and lifestyle changes have been shown to reduce the associated metabolic syndrome (Crist, 2012). When used in conjunction with bariatric surgery, there is improved glucose control with type 2 diabetes (Mingrone, 2012; Schauer, 2012).
PREGNANCY AND OBESITY
Obese women unequivocally have reproductive disadvantages. This translates into difficulty in achieving pregnancy, early and recurrent pregnancy loss, preterm delivery, and a myriad increased obstetrical, medical, and surgical complications with pregnancy, labor, delivery, and the puerperium (American College of Obstetricians and Gynecologists, 2013a). Obesity in pregnancy is also associated with increased health-care utilization and costs (Pauli, 2013). Finally, infants—and later, adults—of obese mothers have correspondingly increased rates of morbidity, mortality, and obesity (Reynolds, 2013).
Obesity results in subfertility due to increased insulin resistance as in polycystic ovarian syndrome. Leptin dysregulation also results in loss of gonadotropin secretory rhythms (Maguire, 2012). Impaired fecundity has been linked to women with a BMI > 30 kg/m2 (Neill, 2001). In 6500 in vitro fertilization-intracytoplasmic sperm injection cycles, Bellver and associates (2010) found that implantation, pregnancy, and livebirth rates were progressively and significantly reduced with each unit of maternal BMI. As discussed in Chapter 8 (p. 157), obesity is associated with increased risk of first-trimester and recurrent miscarriage (Lashen, 2004; Metwally, 2008). In the many overweight and obese women who achieve pregnancy, there is a litany of increased and interrelated adverse perinatal outcomes that are discussed subsequently.
 Prevalence
Prevalence
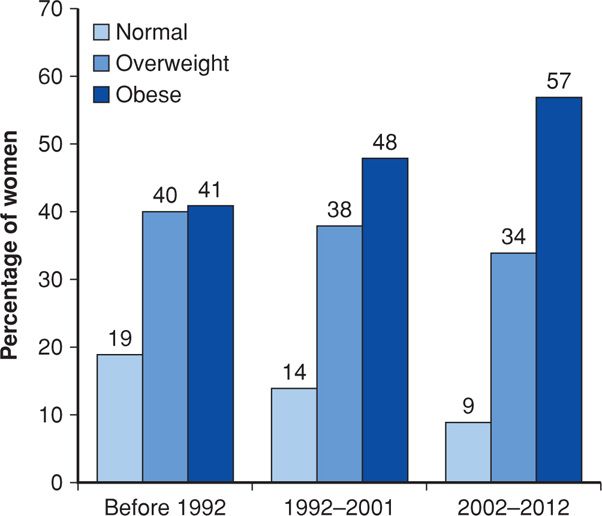
As expected from reviewing Figure 48-2, obesity complicating pregnancy has increased substantially in this country. Before adoption of the BMI, investigators used various definitions of obesity to assess risks during pregnancy. For example, in an earlier study from the University of Alabama at Birmingham, four definitions were used, but regardless of how obesity was defined, all groups showed at least twofold increases in prevalence during the 20-year period (Lu, 2001). Equivalent findings were reported from a 15-year study done in Cleveland (Ehrenberg, 2002). Our experience at Parkland Hospital is similar, as shown in the three epochs depicted in Figure 48-4.
FIGURE 48-4 Increasing prevalence of obesity during three epochs in pregnant women classified at the time of their first prenatal visit at Parkland Hospital. (Data courtesy of Dr. Don McIntire.)
 Maternal Weight Gain and Energy Requirement
Maternal Weight Gain and Energy Requirement
The Institute of Medicine (2009) updated its previous comprehensive reviews of maternal weight gain determinants in relation to biological, metabolic, and social predictors. Its recommended weight gains for various BMI categories are shown in Table 9-5 (p. 177). For overweight women, weight gain of 15 to 25 pounds is recommended. For obese women, the Institute recommends a gain of 11 to 20 pounds. This is because fat deposition is greater in women with high BMIs, and thus, energy costs are significantly lower (Butte, 2004). Kinoshita and Itoh (2006) found that during the third trimester, increases were predominantly in visceral fat. Despite these stores, maternal catabolism is—at least intuitively—not good for fetal growth and development. The American College of Obstetricians and Gynecologists (2013c) has endorsed these Institute guidelines.
These Institute guidelines for obese women have some, although minimal, foundation in scientific evidence (Rasmussen, 2010). In a study of 2080 obese women, there was no advantage to weight gain > 20 pounds (Vesco, 2011). However, Chu and coworkers (2009) reported in the United States in 2004 to 2005 that 40 percent of normal-weight and 60 percent of overweight women gained excessive weight during pregnancy. The Maternal-Fetal Medicine Units Network arrived at similar conclusions. Seventy-five percent of nearly 8300 nulliparas gained more weight than the Institute recommendations (Johnson, 2013). Additionally, women who gained more than the recommended amount had excessive postpartum weight gain at 3, 8, and 15 years after delivery (Nehring, 2011; Rooney, 2002).
 Maternal Morbidity
Maternal Morbidity
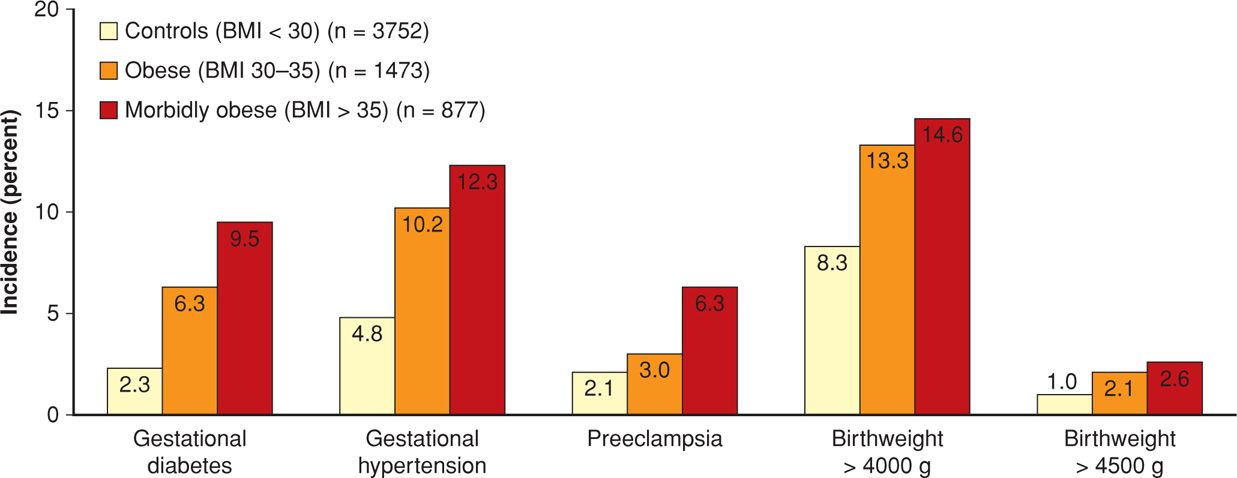
Some of the excess perinatal morbidity due to obesity is listed in Table 48-3. In studies, various definitions of obesity in pregnant women have included 150 percent of ideal body weight, BMI > 35 kg/m2, BMI > 40 kg/m2, BMI > 50 kg/m2, and > 150 pounds over ideal body weight (Cedergren, 2004; Crane, 2013; Denison, 2008; Kabiru, 2004; Kumari, 2001; Stamilio, 2013). In one study, Weiss and colleagues (2004) reported similar adverse outcomes and maternal morbidity in a prospective multicenter study of more than 16,000 women from the FASTER trial (First- and Second-Trimester Evaluation of Risk). As shown in Figures 48-5 and 48-6, especially striking are the marked increases in gestational diabetes and hypertension. Lipkind and associates (2013) showed obesity to be an independent risk factor for “near-miss” maternal morbidity (Chap. 1, p. 6).
TABLE 48-3. Adverse Pregnancy Effects in Overweight and Obese Women
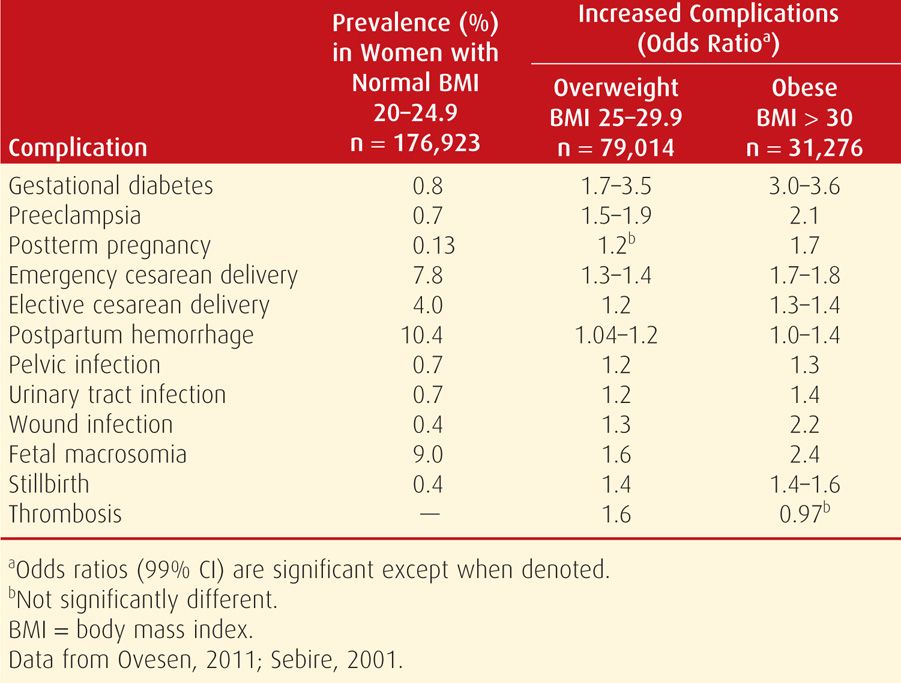
FIGURE 48-5 Incidence of selected pregnancy outcomes in 16,102 women enrolled in the FASTER (First- and Second-Trimester Evaluation of Risk) trial according to body mass index (BMI) status. (Data from Weiss, 2004.)
FIGURE 48-6 Frequency of preeclampsia according to body mass index. (Data from the Hyperglycemia and Adverse Pregnancy Outcome [HAPO] Study Cooperative Research Group, 2010.)
Not shown in Figure 48-5 are the cesarean delivery rates. These were 33.8 percent for obese and 47.4 percent for morbidly obese women compared with only 20.7 percent for the normal-weight control group (Weiss, 2004). Similar results were subsequently described by Garabedian and coworkers (2011). Haeri and colleagues (2009) also found increased rates of cesarean delivery and gestational diabetes in obese adolescents. More worrisome is that obese women also have increased rates of emergency cesarean delivery (Lynch, 2008; Poobalan, 2009). Finally, wound infections are more common. Alanis and associates (2010) reported this complication in 30 percent in women whose BMI was > 50 kg/m2.
There are also reports of increased adverse pregnancy outcomes in overweight women whose BMI is 25 to 29.9 kg/m2 (Hall, 2005). Shown in Table 48-3 were results of two studies that included more than 285,000 singleton pregnancies. Although not as magnified as in the obese cohort, almost all complications are significantly increased in overweight women compared with those whose BMI is normal.
Obesity is detrimental to the accuracy of obstetrical ultrasound examination (Weichert, 2011). Other morbidity includes a higher incidence of failed trial of labor with a prior cesarean delivery (Bujold, 2005; Goodall, 2005; Hibbard, 2006; Robinson, 2005). Obesity and hypertension are common cofactors in causing peripartum heart failure (Cunningham, 1986, 2012). And, obese women present anesthesia challenges that include difficult epidural and spinal analgesia placement and complications from failed or difficult intubations (Hood, 1993; Mace, 2011). Second-trimester dilatation and evacuation was reported to take longer and be more difficult in women whose BMI was 30 kg/m2 or greater (Dark, 2002).
Obese women are less likely to breast feed than normal-weight women (Li, 2003). They also have greater weight retention 1 year after delivery (Catalano, 2007; National Research Council and Institute of Medicine, 2007; Rode, 2005). Finally, there is evidence that quality-of-life measures are negatively affected by obesity during pregnancy (Amador, 2008). LaCoursiere and Varner (2009) found that postpartum depression was significantly increased in obese women in relation to the degree of obesity—class 1, 23 percent; class 2, 32 percent; and class 3, 40 percent.
Gestational Diabetes
Obesity and gestational diabetes are inextricably linked. Their coexistence with and adverse effects on pregnancy outcomes are discussed in Chapter 57 (p. 1139).
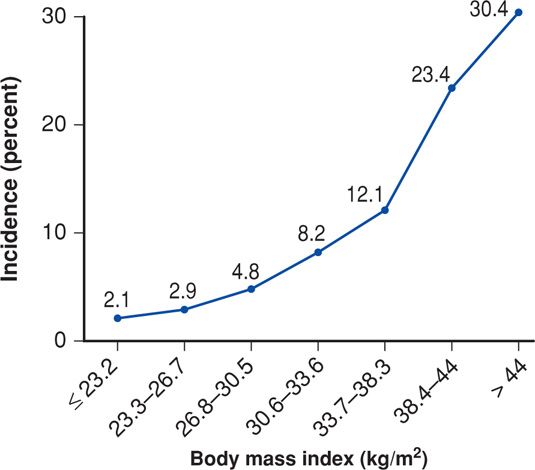
Preeclampsia
There is no doubt that obesity is a consistent risk factor for preeclampsia (see Fig. 48-6). In a review of studies that included more than 1.4 million women, O’Brien and associates (2003) found that the preeclampsia risk doubled with each 5 to 7 kg/m2 increase in prepregnancy BMI. In The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study (2010), the incidence of preeclampsia increased almost geometrically with each BMI category. Obesity and the metabolic syndrome discussed on page 962 are characterized by insulin resistance causing low-grade inflammation and endothelial activation (Catalano, 2010). These have a central and integral role in development of preeclampsia as discussed in Chapter 40 (p. 733). Wolf and colleagues (2001) linked these two conditions, and Ramsay and coworkers (2002) confirmed that obese pregnant women had significantly elevated serum levels of IL-6 and C-reactive protein and impaired endothelial function. Obese gravidas were found to have significantly higher levels of triglycerides, very-low-density lipoprotein cholesterol, insulin, and leptin compared with normal-weight pregnant woman.
Contraception
Most studies report that oral contraceptive failure is more likely in overweight women. This is discussed in detail in Chapter 38 (p. 695).
 Perinatal Mortality
Perinatal Mortality
Stillbirths are more prevalent as the degree of obesity increases (see Table 48-3). Indeed, in a review of almost 100 population-based studies, Flenady and associates (2011) found that obesity was the highest ranking modifiable risk factor for stillbirth. Chronic hypertension with superimposed preeclampsia associated with obesity is one cause of excessive stillbirths. An increased incidence of otherwise inexplicable late-pregnancy stillbirths and early neonatal deaths is also associated with obesity (Cnattingius, 1998; Stephansson, 2001; Waldenström, 2014; Yao, 2014). In one metaanalysis, there was a 1.5-fold increased risk for stillbirth in overweight and a 2.1-fold risk in obese women (Chu, 2007). A Scottish study of more than 186,000 nulliparas described an almost fourfold increased stillbirth rate in women with a BMI ≥ 35 kg/m2 compared with women of normal size (Denison, 2008). Increased prepregnancy weight was the factor most strongly associated with unexplained fetal deaths even after adjusting for maternal age and excluding women with diabetes and hypertensive disorders (Huang, 2000; Nohr, 2005; Ovesen, 2011).
 Perinatal Morbidity
Perinatal Morbidity
Both fetal and neonatal complications are increased in obese women. The Atlantic Birth Defects Risk Factor Surveillance Study found a two- to threefold increased incidence in various anomalies in obese women (Watkins, 2003). Rasmussen and coworkers (2008) performed a metaanalysis and found 1.2-, 1.7-, and 3.1-fold increased risks for neural-tube defects in overweight, obese, and severely obese women, respectively. Another metaanalysis found that maternal obesity was significantly associated with an increased risk of a wide range of anomalies (Stothard, 2009). The National Birth Defect Prevention Study reported a correlation between BMI and congenital heart defects (Gilboa, 2010). According to Biggio and colleagues (2010), however, this may be related to diabetes as a cofactor. Already mentioned is concern for unreliable fetal anatomy sonographic screening in obese women (Dashe, 2009; Thornburg, 2009; Weichert, 2011).
Two important and interrelated cofactors that contribute to excessive rates of perinatal morbidity are chronic hypertension and diabetes mellitus, both of which are associated with obesity. Affected women have increased rates of preterm delivery and fetal-growth restriction (McDonald, 2010; Waldenström, 2014; Wang, 2011). As discussed above, pregestational diabetes increases the birth defect rate, and gestational diabetes is complicated by excessive numbers of large-for-gestational age and macrosomic fetuses (Chap. 44, p. 884).
Even without diabetes, the prevalence of macrosomic newborns is increased in obese women (Cedergren, 2004; Ovesen, 2011). The group from MetroHealth Medical Center in Cleveland has conducted studies of prepregnancy obesity, gestational weight gain, and prepregnancy and gestational diabetes and their relationship to newborn weight and fat mass (Catalano, 2005, 2007; Ehrenberg, 2004; Sewell, 2006). Although each of these variables was found to be associated with larger and more corpulent newborns, prepregnancy BMI had the strongest influence on the prevalence of macrosomic neonates. They attributed this increased prevalence of macrosomic infants to the marked frequency of overweight or obese women in pregnancy—47 percent—rather than the 4 percent of pregnant women with diabetes. More recently, Edlow and associates (2013) have shown that genes expressed in the adult metabolic syndrome may be initiated in the fetus.
Morbidity in Children Born to Obese Women
It appears that obese women beget obese children, who themselves become obese adults. Whitaker (2004) studied low-income children in the Special Supplemental Food Program for Women, Infants, and Children (WIC) and found a linear association between early pregnancy maternal BMI and prevalence of overweight children at 2, 3, and 4 years. Schack-Nielson and associates (2005) reported a direct association between maternal, newborn, and childhood BMI. This association strengthened as offspring progressed to adulthood. Catalano and coworkers (2005) studied offspring at a median age of 7 years and found a direct association with maternal prepregnancy obesity and childhood obesity. They also reported associations with central obesity, elevated systolic blood pressure, increased insulin resistance, and decreased high-density lipoprotein (HDL) cholesterol levels—all elements of the metabolic syndrome. In their analysis of 28,540 women, Reynolds and associates (2013) found increased rates of cardiovascular disease and all-cause mortality in offspring of overweight and obese mothers. Boney and colleagues (2005) studied offspring of women with or without gestational diabetes. They observed that children who were large for gestational age at birth and whose mothers were either obese or had gestational diabetes had significantly increased risks for developing metabolic syndrome.
It also appears that excessive maternal weight gain in pregnancy may predict adulthood obesity in their offspring. Schack-Nielsen and associates (2005) found a linear association of maternal weight gain with the subsequent BMI in their children. Analyzing data from Project Viva, Oken (2006) confirmed this. However, not all studies concur. In their analysis of children in the WIC program cited above, Whitaker and associates (2004) found no obvious linear association between gestational weight gain and childhood obesity. This is also discussed in some detail in Chapter 44 (p. 876).
Fetal Programming and Childhood Morbidity
Epidemiological studies have addressed the association of childhood, adolescent, and even adult adverse health outcomes in relation to the fetal environment. Adverse outcomes include obesity, diabetes, hypertension, and the metabolic syndrome. Variables studied have included maternal prepregnancy BMI, obesity, gestational weight gain, and pregestational or gestational diabetes. The most robust evidence suggests a direct association between children born to women who had prepregnancy obesity or gestational diabetes and being overweight in childhood and adulthood.
The potential biological causes and mechanisms of these associations are not clear. Elucidation is limited by insufficient data on potential maternal and genetic predisposing factors and on the environment of the infant and child in relation to diet and activity. The science of epigenetics has provided some support for the possibility that perturbations of the maternal-fetal environment can adversely alter postdelivery events (Aagard-Tillery, 2006). Perhaps more likely are contributions of the maternal-child environment subsequent to birth (Gluck, 2009). These and other factors regarding fetal programming are discussed in Chapter 44 (p. 876).
 Antepartum Management
Antepartum Management
Dietary Intervention in Pregnancy
Weight reduction is not advisable during pregnancy (Catalano, 2013). As noted, recommended weight gain in obese women is 11 to 20 pounds, and several dietary interventions to limit weight gain to these targets have been reported. These include lifestyle interventions and physical activity (Petrella, 2013). Reviews by Quinlivan (2011) and Tanentsapf (2011) found that randomized trials generally reported successful results with intervention. On the other hand, in many other studies, either these have been unsuccessful or the results were insufficient to permit a conclusion (Campbell, 2011; Dodd, 2010; Guelinckx, 2010; Nascimento, 2011; Ronnberg, 2010). Special attention to psychological aspects of pregnancy has been recommended by some (Skouteris, 2010).
Prenatal Care
Close prenatal surveillance detects most early signs of diabetes or hypertension. Standard screening tests for fetal anomalies are sufficient, while being mindful of sonographic limitations for detection of fetal anomalies. Accurate assessment of fetal growth usually requires serial sonography. Antepartum and intrapartum external fetal heart rate monitoring are likewise more difficult, and sometimes these are even impossible.
 Surgical and Anesthetic Concerns
Surgical and Anesthetic Concerns
Evaluation by the anesthesia team is performed at a prenatal visit or on arrival at the labor unit (American College of Obstetricians and Gynecologists, 2013b). Anesthetic risks and complications faced by obese women are discussed in Chapter 25 (p. 505). Special attention is given to complications that might arise during labor and delivery. Vricella and coworkers (2010) reported the following frequencies of anesthetic complications in 142 morbidly obese women at cesarean delivery. These included technical problems with regional analgesia—up to 6 percent; use of general anesthesia—6 percent; hypotension—3 percent; and overall anesthetic complications of 8.4 percent.
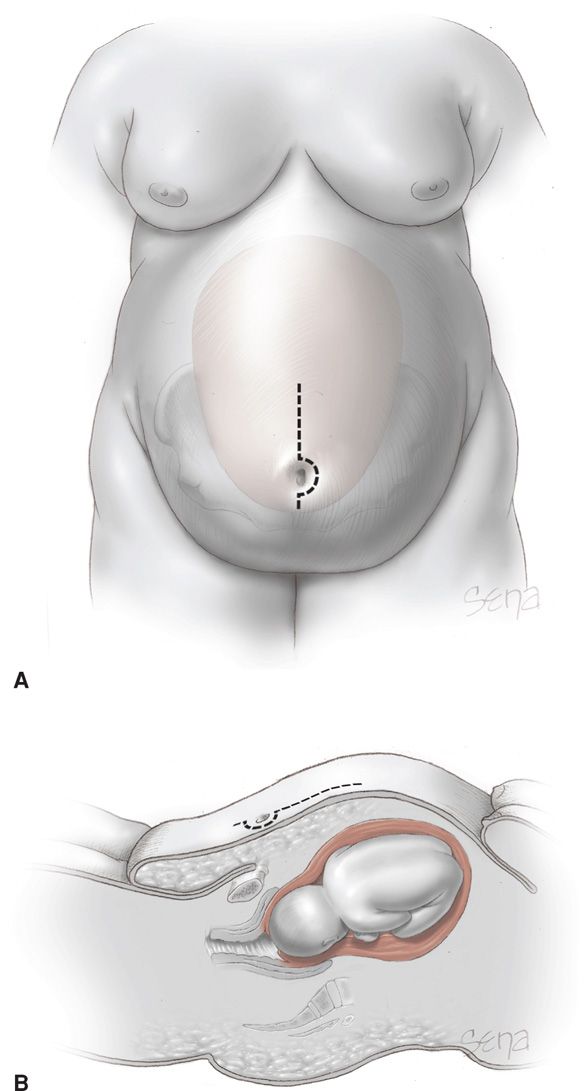
For cesarean delivery, forethought is given to optimal placement and type of abdominal incision to allow access to the fetus and to effect the best wound closure with the least intervening tissue (Alexander, 2006). One technique is shown in Figure 48-7. Others prefer a transverse abdominal incision (Alanis, 2010). Individual differences in maternal body habitus preclude naming any one approach as superior (Gilstrap, 2002; McLean, 2012). Although some have reported similar wound complications with either incision, others reported a fourfold wound complication rate when a vertical abdominal incision was compared with a transverse incision—31 versus 8 percent (Houston, 2000; Thornburg, 2012; Wall, 2003). Finally, a mid-abdomen transverse incision has been advocated by some (Tixier, 2009).
FIGURE 48-7 Abdominal incision for the obese woman. A. Frontal view. The dotted line indicates an appropriate skin incision for abdominal entry relative to the panniculus. As shown by the uterus in the background, selection of this periumbilical site permits access to the lower uterine segment. B. Sagittal view.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree