Normal Morphology of the Female Genital Tract 2
| 2 | Normal Morphology of the Female Genital Tract |
Portio and Vagina
Macroscopy
The entire vaginal epithelium is visualized macroscopically by spreading the elastic vaginal walls with a speculum.
Types of epithelia. The vaginal epithelium is free of glands and pigment, so it appears pale pink in color. Unlike vulvar epidermis, the epithelium of the vagina does not keratinize. The vaginal epithelium extends from the inner thirds of the labia minora to the portio vaginalis. (The abbreviated terms for parts of the uterus are “portio” or “portio vaginalis” for portio vaginalis uteri and “cervix” for cervix uteri.) In the area of the external cervical os (ostium of uterus), the vaginal epithelium turns into the glandular mucosa of the cervical canal (Fig. 2.1). Whereas the transition from the vulvar epidermis to the vaginal epithelium is gradual, the transition from the vaginal epithelium to the cervical mucosa is abrupt. This abrupt transition is characterized by a change in color from pink to red and by a change in surface structure from smooth to papillary. The boundary between the squamous epithelium of the vagina and the columnar epithelium of the endocervix is called the squamocolumnar junction (SCJ) and may be located on the ectocervix or inside the endocervix.
Effect of sex hormones. If the effect of sex hormones is prominent—as it is during sexual maturity and particularly during pregnancy—the junction is located on the ectocervix and therefore easy to see. The intensely reddened mucosa is called ectopia or ectropium (Fig. 2.2). It is sensitive to touch and may easily bleed on contact. After menopause, the boundary between vaginal epithelium and mucosa is usually no longer visible because it has withdrawn into the interior part of the cervical canal under the decreasing influence of the sex hormones.
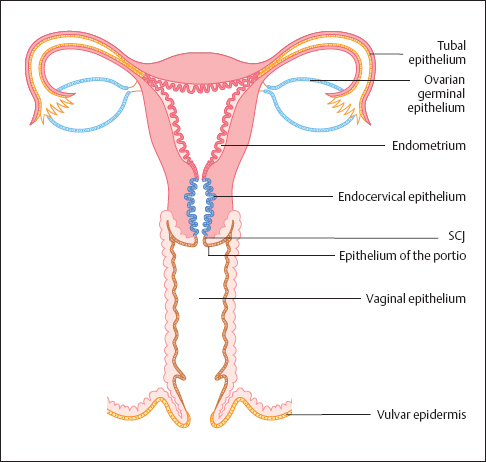
Fig. 2.1 Types of epithelia of the external and internal female genitals. SCJ, squamocolumnar junction.

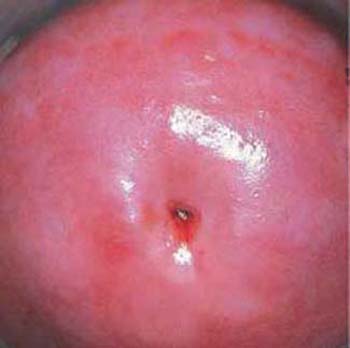
Fig. 2.3 Normal portio vaginalis. The squamous epithelium is smooth and pink. The boundary to the columnar epithelium lies inside the cervical canal and cannot be seen here.
In nulliparous women, the external cervical os has the form of a pit (Fig. 2.3), but in parous women it is a transverse slit, thus forming the anterior and posterior lips of the ostium of uterus (Fig. 2.2).
Structure and function. The portio vaginalis is the cone-shaped lower part of the uterus that extends into the vaginal tube. It serves as an occlusion area for the uterine cavity and is subjected to a complicated opening mechanism during childbirth. The reflections of the anterior and posterior lips of the ostium of uterus toward the vaginal wall are called the anterior and posterior parts of the vaginal fornix. The anterior vaginal fornix lies posterior to the floor of the urinary bladder, and the posterior vaginal fornix lies anterior to the rectum.
Blood flow. The vaginal mucosa consists of an epithelial part and a connective tissue part. The connective tissue is called stroma or dermis; it supplies the epithelium with blood and nutrients (Fig. 2.4). Countless vascularized dermal papillae, a few millimeters apart, extend like cones into the epithelium. The epithelium is thinner over the tips of the papillae than it is between them, and the vascularized papillae appear as a fine reddish punctation when viewed from above at low magnification (Fig. 2.5). Under certain conditions (e. g., acute inflammation), there may be an increase in blood flow through the papillae, thus intensifying the pattern of punctation (see Fig. 3.96 b, p. 107).
Atrophy of the portio vaginalis. An atrophic portio exhibits signs of regression due to an increase in connective tissue, a decrease in blood flow, and a thinning of the epithelial layer (54). In old age, the size of the portio may be reduced to such an extent that it comes to lie at the same level as the cervical mucosa. The vaginal epithelium is pale pink in color and less reflective than during sexual maturity (Fig. 2.6). Contact often causes petechial bleeding, since there is no protective effect of the once highly developed epithelium.
Histology
The nonkeratinized squamous epithelium consists of four layers:
- Basal cell layer (stratum basale)
- Parabasal cell layer (stratum parabasale)
- Intermediate cell layer (stratum spinosum)
- Superficial cell layer (stratum superficiale)
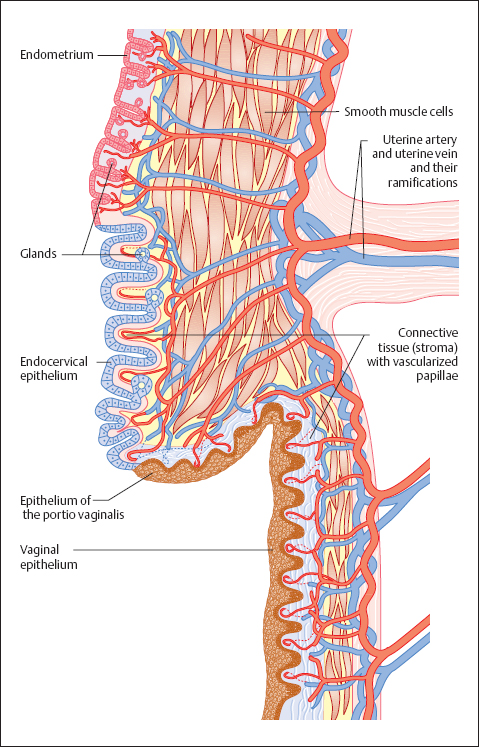
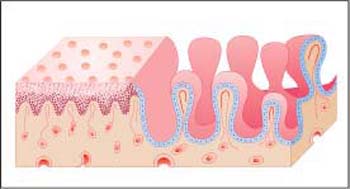
Fig. 2.5 Epithelia of portio and endocervix. Diagram showing the histological structure of the two different epithelial surfaces. The squamous epithelium has a smooth surface with a fine, reddish punctation, while the endocervical epithelium has a villiform surface.
Papillary body. In thin tissue sections, which are normally cut perpendicularly to the surface of the epithelium, the connective tissue part and the epithelial part of the tissue can be clearly differentiated from each other. The blood and lymph vessels run through numerous dermal papillae and bring nutrients to the epithelium. This structure provides a firm anchorage for the epithelium onto the supporting connective tissue. The layer of dermal papillae as a whole is called the papillary body and is separated from the epithelium by a basement membrane. This is a semipermeable barrier at which the exchange of metabolites between dermis and epithelium takes place (191). Leukocytes can also penetrate this membrane (345). Immediately on top of the basement membrane is the basal cell layer of the epithelium, and the basement membrane is thought to be a product of the basal cells (284). In the case of epithelial atrophy, the papillary body is visibly flattened.
Epithelial structure. The squamous epithelium of the vagina is multilayered and—depending on hormonal influences—consists of 5–50 cell layers (390) that do not keratinize at the surface. The vaginal epithelium is therefore a stratified, nonkeratinized squamous epithelium (Fig. 2.7).
- Basal cell layer. The lowest stratum of the epithelium is a single layer of small, cuboidal basal cells, each with a large nucleus that often contains a nucleolus. These cells are able to divide, and they respond to numerous stimuli with increased proliferation (266).
- Parabasal cell layer. The parabasal cells form about 5-10 layers on top of the basal cell layer; they are slightly larger than basal cells and have a large nucleus that sometimes contains a nucleolus. The cytoplasm stains dark because it is rich in RNA.
Several studies suggest that the actual renewal of cells does not originate from the basal cells but from small, deep-seated parabasal cells (333). These are pluripotent cells that produce a stratified squamous epithelium under normal conditions; under certain conditions, however, they may follow other pathways of differentiation. It is thus possible that they develop either into reticular cells, especially histiocytes, or into regenerative epithelial cells, or even into glandular cells (355).
Once these cells have developed into large, more superficial parabasal cells, differentiation into a squamous epithelium is no longer reversible (375, 377). The layers of basal cells and deep-seated parabasal cells represent the germinative center for cell renewal (stratum germinativum), which gives rise to all cell layers above. According to their topographic position, these layers are called the parabasal, intermediate, and superficial cell layers.
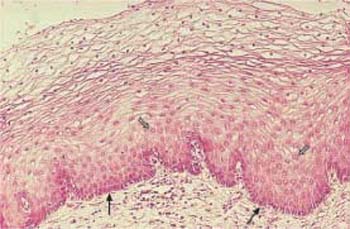
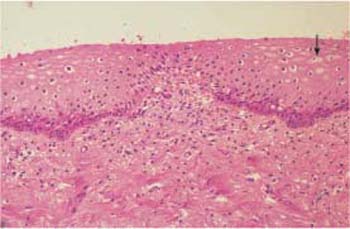
Fig. 2.8 Atrophic vaginal epithelium after the menopause. The papillary body is markedly flattened, and the epithelium consists of at most 10 cell layers. Above the dark basal cell layer, the epithelium contains almost exclusively parabasal cells. Isolated intermediate cells are visible at the surface (→). HE, ×100.
- Intermediate cell layer. With increasing maturity, the cells become larger and produce glycogen, which becomes incorporated into their cytoplasm (85). The intermediate cells formed in this way occupy a space about 15–30 cell layers thick. Because of the increasing pressure, the cells flatten. The cytoplasm is light, and the nucleus is markedly smaller than in the parabasal cell layer.
- Superficial cell layer. This layer is about 510 cell layers thick. Under the influence of estrogen, mucopolysaccharides are formed instead of glycogen (85) and provide the cells with a resistant, keratin-like scaffold. At the same time, the nucleus degenerates and shrinks (pyknosis).
- Process of cell maturation. The uppermost cell layers may easily peel off (exfoliation) because the intercellular adhesion sites (desmosomes) are progressively reduced during epidermal cell maturation (379). The entire intercellular space contains tissue fluid which enables both the transport of substances and the migration of leukocytes. The superficial and intermediate cells exfoliate into the vaginal cavity. The sugar molecules contained in the glycogen of exfoliated cells are degraded into lactic acid by the Doderlein’s bacilli of the vagina (vaginal lactobacilli) (410), and the cells dissolve (cytolysis). The entire process of maturation lasts less than a week under normal conditions during sexual maturity.
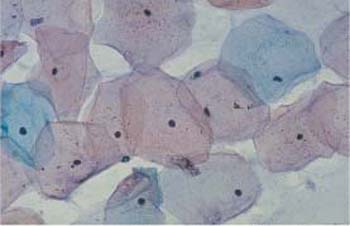
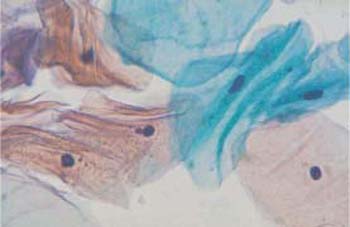
Fig. 2.10 Superficial cells. Wrinkled superficial cells with pyknotic nuclei. The cells are partly eosinophilic, partly basophilic. ×790.
Epithelial atrophy. In the absence of sex hormones after the menopause, the squamous epithelium consists of only a few cell layers (Fig. 2.8). A similar situation exists during childhood, during the postpartum period, and in certain endocrinological diseases associated with a greatly reduced production of sex hormones.
Cytology
The gynecological smear shows four types of squamous epithelial cells:
- Superficial cells
- Intermediate cells
- Parabasal cells
- Basal cells
In the cytological smear, the cells are no longer associated to form a tissue. Instead, the exfoliated cells lie isolated, flattened, and spread out. Hence, they are viewed from above, which makes them appear larger and more voluminous than when they are viewed in histological sections. Individual cells can be better assessed in this way.
Smears from the female genital tract almost exclusively undergo staining according to the method introduced by Papanicolaou (see p. 339).
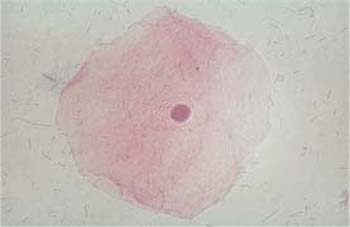
Superficial cells. Superficial cells are flat and stain either basophilic or eosinophilic. They are polygonal in shape, with a diameter of 50–60μm. Their cytoplasm is transparent, homogenous, and has well-defined margins. The cells are more spread out during the first half of the menstrual cycle and appear more wrinkly during the second half (Figs. 2.9, 2.10) (88). The cells occur isolated or, especially during the second half of the cycle, in loose clusters where individual cell margins are always well defined. Occasionally, keratohyalin granules are found in the cytoplasm (Fig. 2.11a). The cells have either a large, active, vesicular nucleus of about 7 urn in diameter with easily recognizable, fine, and evenly dispersed chromatin (Fig. 2.11b), or a shrunken pyknotic nucleus with condensed, darkly staining chromatin due to degeneration (Fig. 2.11c). Due to the effect of estrogen, eosinophilic superficial cells with pyknotic nuclei dominate at mid-cycle, and basophilic superficial cells with active, vesicular nuclei dominate at the beginning and at the end of the cycle (293).
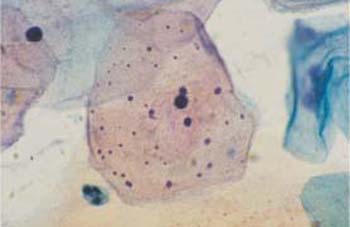
b Cell with a polygonal shape, clearly defined cell margins, and an active, vesicular nucleus.
c Cell with a degenerated, pyknotic nucleus.
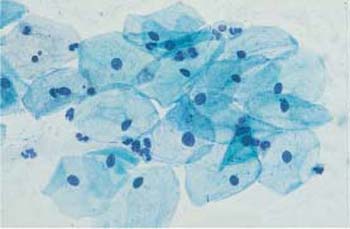
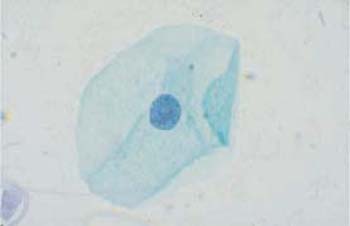
Fig. 2.13a, b Intermediate cells. Each micrograph shows a flat, basophilic intermediate cell with the cytoplasm spread out. ×1000.
a Cell with a polygonal shape, clearly defined cell margins, and an active, vesicular nucleus.
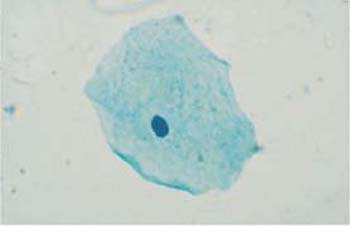
b Cell with a degenerated, pyknotic nucleus.
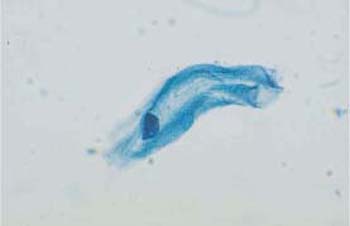
Fig. 2.14 Intermediate cell. This cell has a wrinkled cytoplasm and a pyknotic nucleus. ×1000.
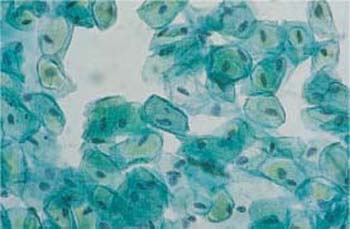
Fig. 2.15 Navicular cells. Boat-shaped intermediate cells with wrinkled cytoplasm and thickened cell margins are called navicular cells. ×400.
Intermediate cells. These are about 30-50 um in diameter and have a large nucleus about 8 um in diameter. They have basophilic staining characteristics and are polygonal or round in shape. Their nuclei are usually active but sometimes also pyknotic (Fig. 2.12, 2.13a, b). During the second half of the cycle, the intermediate cells tend to form clusters and have wrinkles like the superficial cells (Fig. 2.14). We distinguish between large and small intermediate cells; the large ones already contain glycogen, but the small ones do not. During pregnancy, and under the strong influence of gestagen or androgen, these cells are often boat-shaped, with thickened cell margins. They are therefore called navicular cells (271) (Fig. 2.15).
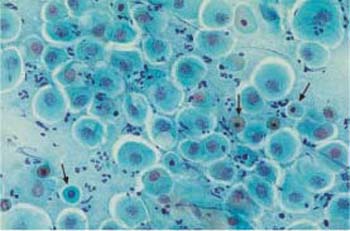
b Cells with degenerated (condensed) nuclei.
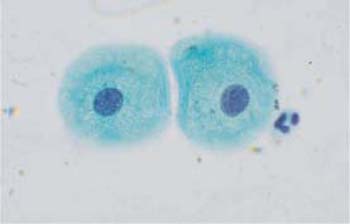
Fig. 2.17 Two parabasal cells with rounded cytoplasm and active vesicular nuclei. ×1000.
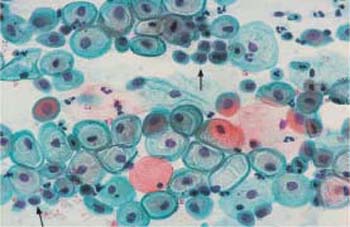
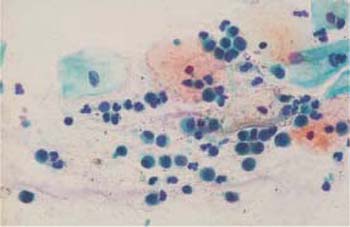
Fig. 2.19 Basal cells with rounded cytoplasm and partly active, partly degenerated nuclei. Some superficial and intermediate cells are also visible. ×400.
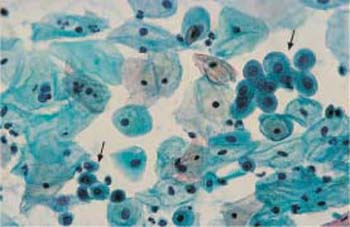
Fig. 2.20 Basal cells. Two clusters of basal cells (→) with rounded cytoplasm and pyknotic nuclei, surrounded by parabasal and intermediate cells. ×400.
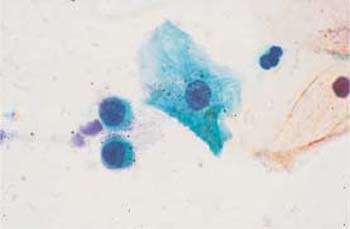
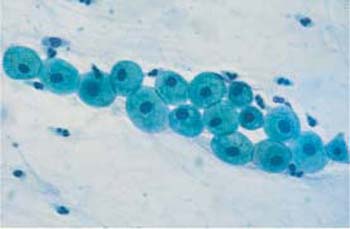
Parabasal cells. Parabasal cells are about 20 um in diameter and have a nucleus of about 9 um in diameter. They are strongly basophilic and have an oval to round shape (Fig. 2.16a, b, 2.17). There are small and large superficial parabasal cells. After the menopause in childhood, and in the postpartum period, and also in certain endocrine diseases, they are the dominant cell type in the smear. They often show degenerative changes due to their low resistance (81). In the postpartum period, they incorporate glycogen which makes them more resistant; they frequently have thickened, well-defined cell margins and a nucleus that sometimes appears displaced toward the edge of the cell. These cells are called postpartum cells (Fig. 2.18).
Basal cells. These cells have a diameter of 12–14 μm and a nuclear diameter of 8–10 μm, i. e., the nucleocytoplasmic ratio is clearly shifted toward the nucleus (Fig. 2.19). The cells are round and show strong basophilic staining, and their nuclei are often degenerate (Fig. 2.20). If the chromatin structure is still intact, a nucleolus may be visible (Fig. 2.21). In severe epithelial atrophy, the basal cells exfoliate into the vaginal cavity. They are also regularly found during the reparative phase of inflammatory processes; because the basal cell hyperplasia accompanying the inflammation causes a thickening of the basal epithelial layer, the cells come to the surface (81).
Hormonal Cytology
The nonkeratinized squamous epithelium is primarily the target organ of sex hormones, but other hormones and hormone-like substances also influence the epithelium:
- Estrogens (estradiol, estriol, estrone), which are follicular hormones
- Gestagens (progesterone), which are corpus luteum hormones
- Other hormones (androgens, corticoids, gonadotropins, etc.)
The sex hormones have a decisive effect on the structure and thickness of the vaginal squamous epithelium. The composition and the activity levels of these hormones are determined by the woman’s age, possible hormonal therapies or replacement, and also by pregnancy and lactation (385).
Cell indices and Schmitt staging are used to determine the degree of cellular proliferation.
Mechanism of action of sex hormones. The stimulating effect of sex hormones on the proliferation of the vaginal squamous epithelium is triggered either by endogenous production or by exogenous supply (Fig. 2.22). The type of supply is not important in achieving an effect. Application of hormones may be oral, parenteral, transcutaneous, or topical, although topical intravaginal application has the fastest and strongest effect (210).
The activity of sex hormones is based on their direct binding to a receptor molecule on the cell membrane. The activated receptor is then transported to the nucleus, where the hormone induces DNA synthesis within a few minutes (162, 163). If the hormonal effect continues, it takes about a week for the epithelium to develop from a state of complete atrophy to a fully differentiated epithelial structure (368). Estrogens are considerably more effective than gestagens or androgens; the latter induce only a moderate level of proliferation of the squamous epithelium when given on their own (419).
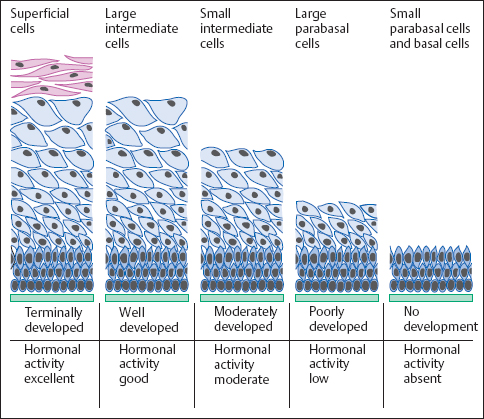
Fig. 2.22 Vaginal epithelium. Diagram showing the changes in histological structure depending on the activity of sex hormones.

Fig. 2.23 Vaginal epithelium. Diagram showing the changes in histological structure depending on the phase of the menstrual cycle.
Age-dependent differences. In a sexually mature woman, the vaginal epithelium is normally highly developed, containing not only the basal and parabasal cell layers but also the intermediate and superficial cell layers (400, 401). In childhood, during lactation, and after the menopause, the epithelium develops only as far as producing a parabasal cell layer, since stimulation by sex hormones is absent during these periods of life.
Phases of the menstrual cycle. During the normal cycle, the thickness of the epithelium differs depending on the hormonal situation (Fig. 2.23). At the beginning and end of the cycle, epithelial development proceeds only to the upper intermediate cell layer, whereas at midcycle, when estrogen activity is at its peak, the epithelium is fully developed and includes a superficial cell layer. The phases of the ovarian cycle (follicular phase, luteal phase, and menstrual phase) are reflected by the cells present in the vaginal smear (297).
- During the follicular phase, which lasts from about day 5 to day 15 of the cycle, the cells are predominantly isolated, flat, and spread out (Fig. 2.24). The number of eosinophilic superficial cells with nuclear pyknosis peaks on the day of ovulation, and cytolysis stops (Fig. 2.25). At this point, the accompanying cell image is usually very clean and free of leukocytes (291). At the same time, the cervical mucus liquefies; clinically, it shows an increase in threadability and a special crystallization pattern, called fern-leaf artifact, in native preparations and occasionally also in stained preparations (Fig. 2.26)(274).
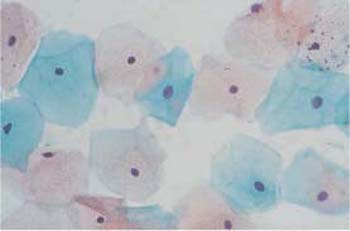
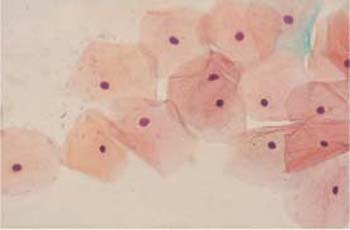
Fig. 2.25 Ovulation phase. Flat, spread-out eosinophilic superficial cells with pyknotic nuclei. ×400.
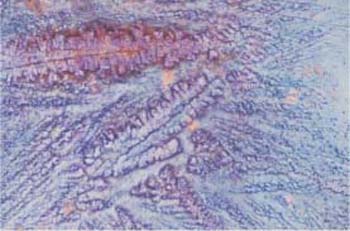
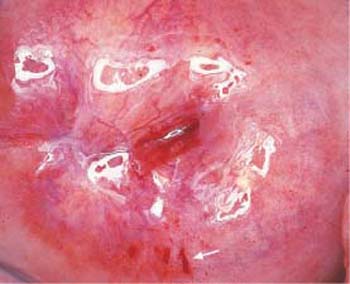
Fig. 2.26 Fern-leaf phenomenon. Crystallization pattern of the cervical mucus during the ovulation phase. ×125.
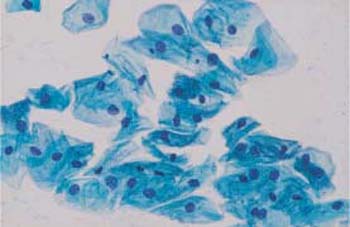
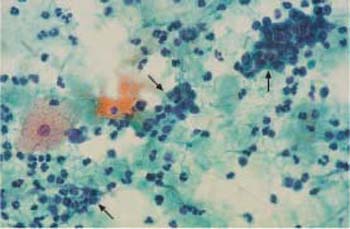
Fig. 2.28 Menstrual phase. The background of the preparation suggests inflammation. Endometrial cells (→) and leukocytes. ×400.
- During the luteal phase (days 15–28), when the cells are under the influence of progesterone, they often lie in clusters, and wrinkles or rolled-in margins can be seen in the cytoplasm (Fig. 2.27) (298). At the same time, the number of cyanophilic intermediate cells with active, vesicular nuclei rises again, indicating cytolysis. The accompanying cells include increasing numbers of leukocytes and histiocytes.
- Beginning with the menstrual phase (days 1–5), huge numbers of erythrocytes as well as endometrial and stromal cells are also present. Hence, the background of the preparation appears dirty and inflammatory (Fig. 2.28).
Cell indices. To determine the effects of estrogen and gestagen on the squamous epithelium, cell indices are used to report the percentage of mature or wrinkled superficial cells (320).
- The karyopyknosis index gives the percentage of pyknotic nuclei of squamous epithelial cells.
- The eosinophilia index gives the percentage of superficial cells that are stained red (215).
- The wrinkle index gives the percentage of wrinkly superficial and intermediate cells (408).
The eosinophilia index is about 20% at the beginning and the end of the cycle and reaches 50% at midcycle. The karyopyknosis index is always about 10% higher than the eosinophilia index, and follows a similar cyclic course. The curves of both indices correspond to estradiol concentrations in the serum—the only difference being that the hormone peaks 1–2 days before the indices reach their maxima (260). The squamous epithelium requires this latency period in order to respond to the hormonal changes.
The influence of ovulation inhibitors. The spontaneous course of the cycle may show variations, depending on the length of the cycle and whether or not ovulation has occurred. The course is also altered by intake of ovulation inhibitors, since the production of sex hormones in the ovary is artificially suppressed (199, 280). By means of a feedback mechanism, the administration of hormones leads to the inhibition of gonadotropin release from the hypophysis, since the production of folliclestimulating hormone (FSH) and luteinizing hormone (LH) is slowed down. Both these hormones are responsible for the normal spontaneous course of the cycle (370). The dosage and type of application of ovulation inhibitors differ from preparation to preparation:
- For sequential preparations, the normal cycle is imitated by administering the hormones at different times. However, the peak values of cell indices lie clearly below those of the spontaneous cycle (279).
- For combination preparations, the same dose of hormones is administered throughout the cycle, and cell indices remain at a constant low level of 20–30% (202).
- For continuous administration of low doses of gestagens, in the form of the minipill

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


