FIGURE 12.1 (A) Axial head computed tomography (HCT) with left frontal acute intraparenchymal hemorrhage (IPH, white arrow) and secondary intraventricular hemorrhage. (B) Axial fluid-attenuated inversion recovery sequence magnetic resonance imaging (MRI) from the same patient as in (A) at a different level with IPH (white arrow) and arteriovenous malformation at the posterior aspect of the hematoma (white arrowhead).
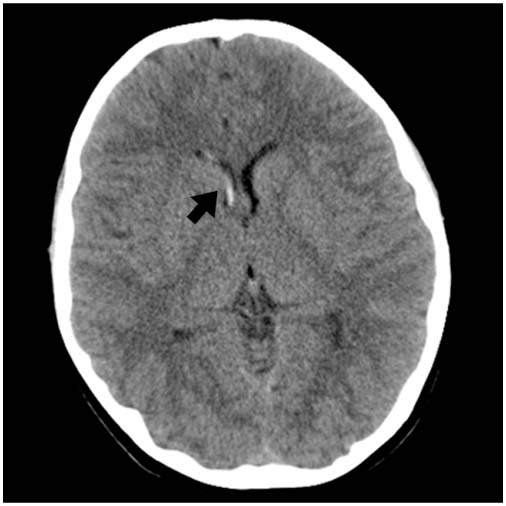
FIGURE 12.2 Axial HCT with intraventricular hemorrhage (black arrow) in the right lateral ventricle without hydrocephalus.
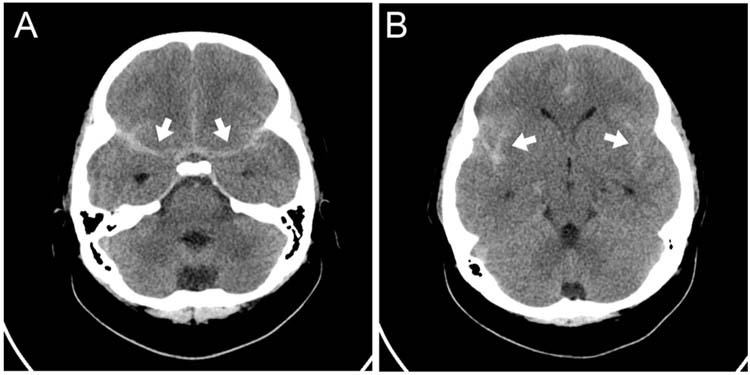
FIGURE 12.3 (A) Axial HCT with subarachnoid hemorrhage visible in bilateral basal cisterns (white arrows). (B) Axial HCT at a different level from the same patient as in A with SAH visible in the bilateral sylvian fissures (white arrows).
■ EPIDEMIOLOGY
Estimates of the incidence of ICH in developed countries range from 1.1/100,000/year to 5.22/100,000/year (1,2). ICH is comprised of intraparenchymal hemorrhage (IPH) and intraventricular hemorrhage (IVH). SAH is generally discussed separately. A California population-based cohort estimated the incidence of SAH to be 0.4/100,000/year. ICH and SAH may occur in the context of traumatic brain injury, but this chapter will focus on nontraumatic causes.
Unlike adult ICH, in which hypertension and amyloid angiopathy are the most common etiologies, childhood ICH is most commonly caused by ruptured vascular malformations including arteriovenous malformations (AVMs) (Figure 12.4), cavernomas (Figure 12.5), and aneurysms (Figure 12.6). Depending on the series, vascular malformations are responsible for 37.5% to 73.5% of pediatric ICH (3,4), with AVM the most common type of malformation. The prevalence of intracranial aneurysms in the pediatric population ranges from 0.5% to 4.6%, and the majority of these patients do not present with acute rupture (5–11).
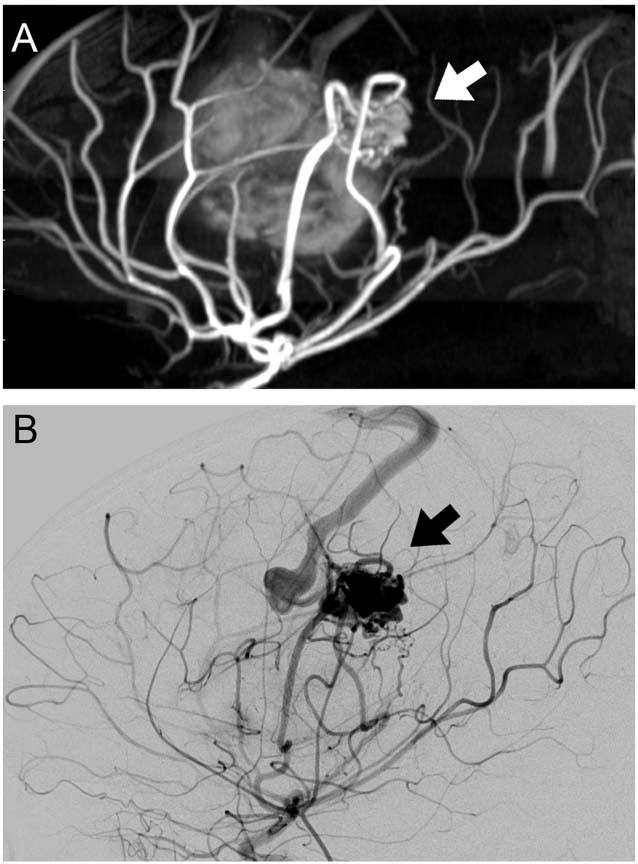
FIGURE 12.4 (A) Sagittal magnetic resonance angiography demonstrating arteriovenous malformation (AVM; white arrow). This AVM was the cause of the IPH shown in Figure 12.1. (B) Sagittal view of digital subtraction angiogram from the same patient demonstrating AVM supplied by distal branches of the left middle cerebral artery (black arrow).
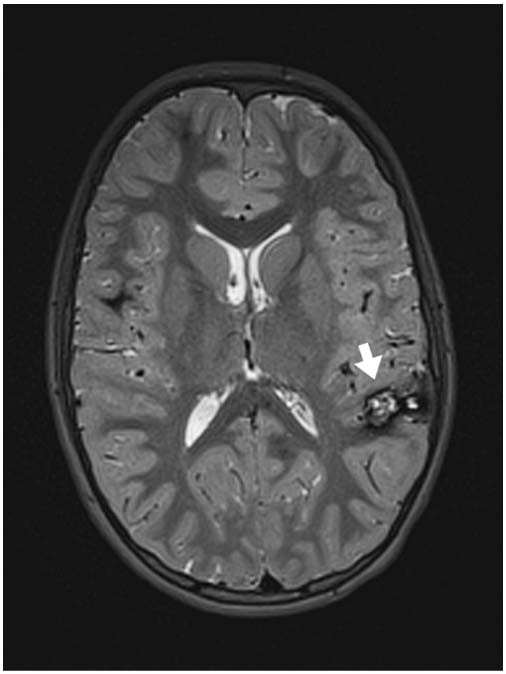
FIGURE 12.5 Axial T2-weighted MRI sequence with left parietotemporal cavernoma (white arrow).
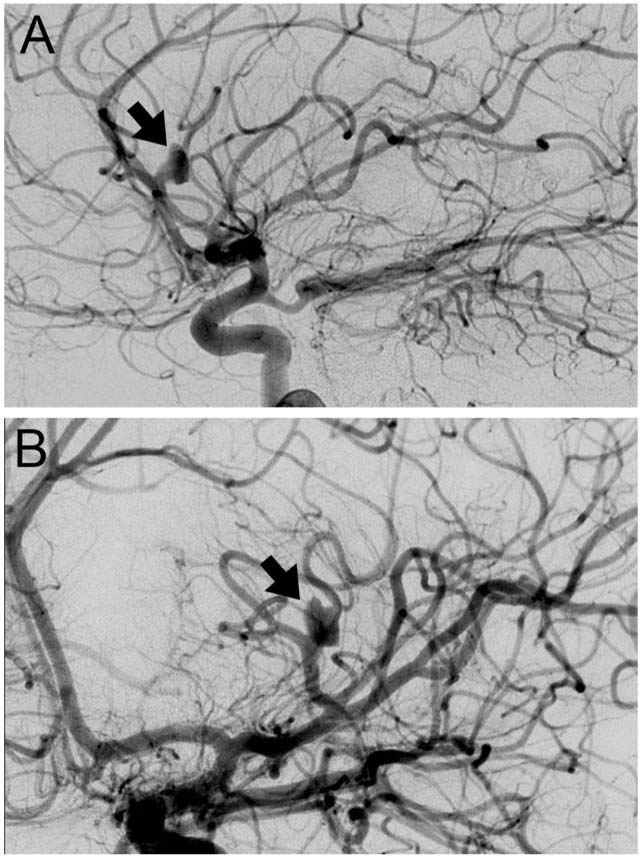
FIGURE 12.6 (A) Digital subtraction angiogram lateral view demonstrating left middle cerebral artery aneurysm (black arrow). (B) Digital subtraction angiogram posterior anterior view of the same patient demonstrating left middle cerebral artery aneurysm (black arrow).
Other common etiologies of ICH in children are hematologic abnormalities and coagulopathies, brain tumors, and cerebral infections (3,12). Although hypertension as a risk factor for ICH is less common than in adults, hypertension has also been associated with ICH in children (12,13). In recent cohorts, even after a thorough evaluation, between 9% and 23% of childhood ICH is considered idiopathic, although some of these cases may be due to vascular malformations that self-obliterate after the incident hemorrhage (12,14). Some children have underlying diseases that predispose them to ICH and/or to vascular malformations and are discussed in the section on special populations.
■ DIFFERENTIAL DIAGNOSIS AND PRESENTATION
The differential diagnosis for ICH is broad and is found in Table 12.1. Presentations of children with ICH can be rapid, occurring over minutes to hours, or insidious over several hours to days (13). In a recent cohort of children with IPH, the median time to hospital presentation was 70 minutes, but 23% of children presented after 24 hours (14). Headache was the most common symptom in most series, affecting 45.6% to 77% of children (3,14). Altered level of consciousness was present in more than 50% of children in several series (3,12,14). Other common presenting symptoms include nausea and emesis, seizures (generalized or focal), neck pain, and focal neurologic deficits such as hemiparesis or aphasia (3,12,14). Common presenting symptoms for SAH also include severe headache, nausea, emesis, neck pain, meningismus, altermental status, and sometimes focal neurological deficits.

■ INITIAL EVALUATION AND MANAGEMENT
Acute studies in the emergency room or intensive care unit should be focused on rapid diagnosis of the hemorrhage, assessment for the presence of elevated intracranial pressure (ICP) and of herniation, and assessment of easily correctible risk factors such as thrombocytopenia or coagulopathy. In most cases, head computed tomography (HCT) is the initial imaging of choice. It is rapid, widely available, can be performed without sedation if the child has preserved airway reflexes and can protect his airway, and clearly diagnoses acute ICH.
In adult series, HCT can miss up to 5% of acute SAH (15–17). Therefore if SAH is suspected and a HCT does not demonstrate hemorrhage, a lumbar puncture should be performed in most cases. A spun down sample of cerebrospinal fluid (CSF) can be examined for the presence of xanthochromia (yellow discoloration of CSF secondary to bilirubin) to differentiate SAH from venous blood from a “traumatic tap.” When sending a CSF sample for suspected SAH, it is important to indicate in the lab order that one wants to check for xanthochromia. A guideline for the analysis of lumbar puncture in adults with possible SAH from the United Kingdom states that if concern for SAH remains after a negative HCT, a lumbar puncture can be performed > 12 hours from symptom onset and the fourth tube sent for spectrophotometry for bilirubin (18). An increase in CSF bilirubin supports the diagnosis of SAH although it is not specific for this diagnosis. Adjustment for elevated serum bilirubin may be necessary (19). Additionally, cell counts can be sent from the first and fourth tubes, since the red blood cell count should decrease between the first and fourth tubes in a traumatic tap whereas the red blood cell count should not decrease between the first and fourth tubes in SAH. However, the magnitude of the decrease in red blood cells that can be used for this differentiation is controversial.
Acute laboratory studies include electrolytes, complete blood count with platelets, prothrombin time or international normalized ratio, activated partial thromboplastin time, and fibrinogen level. A type and screen should be sent as well.
The acute management of any child with ICH or SAH is focused on stabilization of the patient, detection and management of elevated ICP and herniation, and minimizing additional brain injury. No randomized trials exist for childhood ICH or SAH management, so most pediatric guidelines are adapted from those for adults (20–22) or are based on retrospective cohorts.
The patient should not be permitted to eat or drink, even when mental status is preserved since level of consciousness can deteriorate, and intubation or neurosurgical intervention may be required. Once ICH is diagnosed, a neurosurgeon should be contacted immediately. Consideration should be given to consultation with an interventional radiologist for diagnostic and/or therapeutic intervention. The head of the patient’s bed should be elevated to at least 30° and the neck should be maintained in a midline position to promote venous drainage. Signs and symptoms of elevated ICP should be reassessed frequently (see section “Monitoring for Increased ICP”). For any neurologic deterioration, a repeat HCT should be obtained promptly to assess for worsening hemorrhage, hydrocephalus, edema, or herniation.
Isotonic fluids without glucose should be started immediately to maintain adequate hydration (20). Since temperature elevation > 37.5°C increases the likelihood of poor outcome in adult IPH (23), maintenance of normothermia with acetaminophen and cooling blankets is advised. Children who present with seizures should receive an anticonvulsant medication to reduce the likelihood of additional seizures.
Managing hypertension after ICH is challenging since no clear evidence exists in children. In adults with ICH, treatment of elevated blood pressure may prevent expansion of the cerebral hematoma (24), and the adult ICH guidelines discuss management of hypertension (20). Logically, children with hypertension should be treated, and it is a reasonable goal to lower blood pressure to the 95th percentile for age and sex. However, this recommendation is not evidence-based. While lowering blood pressure may reduce the risk of hematoma expansion, it may reduce cerebral perfusion and thus exacerbate secondary brain injury if autoregulatory mechanisms are disturbed or if there is elevated ICP. If there is concern that hypertension is an autoregulatory response to maintain cerebral perfusion or that hypertension is refractory to initial management, ICP monitoring to allow management of ICP and cerebral perfusion should be considered. Antihypertensive medications should be chosen based on intracranial compliance. Vasodilators can reduce systemic blood pressure yet compromise cerebral perfusion pressure. Beta blockade may be helpful in maintaining cerebral perfusion pressure without the concomitant cerebral vasodilation.
For patients with hematologic abnormalities, a pediatric hematologist should be consulted. Patients with low platelet count or abnormal platelet function may require platelet transfusion. Patients with factor deficiencies such as factor XIII or IX deficiency usually require factor concentrate (25,26). Coagulopathy should be corrected for children with abnormal coagulation parameters secondary to liver failure or other medical causes. Children with ICH while on anticoagulation should have their anticoagulation reversed unless there is an overwhelming contraindication such as ongoing extracorporeal membrane oxygenation.
Recombinant activated factor VIIa (rFVIIa) was first used in patients with hemophilia who had developed inhibitors against coagulation factors. rFVIIa has been used in a variety of circumstances in neonates and children who do not have hemophilia, and the mechanism of action is induction of coagulation at sites in which tissue factor is present. However, its safety and efficacy in pediatric ICH has not been studied (27). In adults, a recent meta-analysis underscored that while rFVIIa reduces change in ICH volume, there has not been improvement in outcome or survival (28). Furthermore, there is an increase in thromboembolic events after rFVIIa. Adult studies of rFVIIa may have limited applicability in pediatric patients in whom vascular malformations are the most common cause of ICH since these patients were excluded from adult trials. Additionally, most pediatric patients lack the underlying cardiac and atherosclerotic risk factors that many adults with ICH possess. Other factor concentrates such as prothrombin complex concentrates have been studied in adult ICH in the setting of oral anticoagulation therapy to limit bleeding, but studies in children are lacking (29).
■ ACUTE MEDICAL AND SURGICAL MONITORING AND MANAGEMENT
Monitoring for Increased ICP
Declining mental status in a child with ischemic stroke or ICH is a worrisome sign and is a clear indication for ICP monitoring. Other symptoms and signs of increased ICP include positional headache (headache worse when supine, better when upright), vomiting, irritability or combativeness, sixth nerve palsies, and papilledema. Cushing’s triad suggests elevated ICP and consists of hypertension, bradycardia, and irregular respirations. Cushing’s triad is usually a late finding. With arterial ischemic stroke, increased ICP may develop several days after stroke onset as infarcted brain tissue becomes edematous. In ICH, increased ICP may occur acutely due to mass effect from a hemorrhage. Increased ICP may also occur acutely or subacutely if there is IVH accompanied by communicating hydrocephalus.
If monitoring is required, then an intraventricular catheter (IVC) is advantageous because it provides both a means to measure ICP and to manage ICP via drainage of CSF or blood in the case of IVH. IVC placement requires ventricular enlargement, so it is not an option for all ICH patients. In a recent series of children with IPH, 27% required a ventriculostomy (14). If an IVC cannot be placed due to size of the ventricles or other technical reasons, a subdural bolt can be placed for ICP monitoring. Brain tissue oxygen monitors can be placed to measure oxygen partial pressure, but experience using these types of monitors in pediatric ICH is limited.
Medical Management of Increased ICP
Nonsurgical methods for acutely lowering elevated ICP include keeping the head of the patient’s bed at 30° to promote good cerebral venous drainage, hyperventilation to a PCO2 of 25 to 30 mmHg, and hyperosmolar therapy to promote osmotic diuresis with either mannitol or hypertonic saline. Plasma osmoles and electrolytes must be monitored frequently when osmotic agents are used to avoid hypovolemia, hypotension, and renal failure. In some cases, sedation may be required to help manage elevated ICP. Hyperventilation and hyperosmolar therapy are generally temporizing measures. Corticosteroids should be avoided since adult randomized trials on ICH have not demonstrated efficacy (30,31). Hyperglycemia resulting from corticosteroids has been associated with worse outcome in adults (32,33). For ICH in the setting of a brain tumor, corticosteroids may be considered to limit edema surrounding the tumor.
Surgical Management of Increased ICP
Evacuation of Parenchymal Hemorrhage
While not studied in children, young adults with lobar hemorrhages with clinical deterioration due to mass effect have been reported to benefit from early surgical evacuation in a small retrospective series (34). The Surgical Trial in Intracerebral Hemorrhage (STICH) demonstrated that in adults with spontaneous supratentorial ICH, emergent surgical evacuation of hematoma within 72 hours of bleeding onset did not improve outcome beyond best medical management (35). However, few young patients were enrolled in this trial and children may require more urgent intervention to reduce ICP since they do not have cerebral atrophy that permits expansion of the hematoma. In adults, the revised 2010 guidelines for spontaneous ICH management state that patients with cerebellar hemorrhage, with clinical deterioration, with brainstem compression, or with obstructive hydrocephalus from compression of the ventricles should undergo surgical hemorrhage evacuation (20). Although this scenario has not been specifically studied in pediatric patients, children with cerebellar ICH may require evacuation to prevent herniation (36).
Hemicraniectomy
In adults, timely decompressive craniectomy may be both lifesaving and function-sparing when there is rapid deterioration in the setting of a large arterial ischemic stroke or ICH (37–39). A recent pooled analysis of the three European randomized controlled trials of hemicraniectomy for malignant middle cerebral artery (MCA) infarction demonstrated that hemicraniectomy is a lifesaving procedure and can result in a favorable functional outcome when offered early in adults less than 60 years of age (39).
There are no formal studies of hemicraniectomy in children. In a recent case series of 10 children with malignant MCA infarction, seven underwent hemicraniectomy and all survived and had moderately good recovery (all had hemiparesis but were able to walk and had fluent speech despite left-sided infarcts) (40). The three children who died in this series did not have hemicraniectomy and died of elevated ICP. Time to surgery ranged from 18 to 291 hours, much longer than in adult studies where time to surgery was generally < 48 hours (39). The Glasgow Coma Scale score in children who survived ranged from 4 to 9 suggesting decompression may be beneficial even with deep coma. Although there is no class 1 evidence, some neurosurgeons will consider decompressive hemicraniectomy in patients with a large ICH and declining exam to reduce the likelihood of further deterioration. In a recent small series, 3 of 22 children with ICH had decompressive craniectomy, and all were functionally independent (14).
Monitoring for and Treatment of Seizures
Seizures are a common complication of stroke. Up to 20% of children with ICH will have a seizure (41). Prophylactic anticonvulsants are often used in the setting of ICH or SAH; however, the American Heart Association pediatric stroke guidelines recommend against prophylactic anticonvulsant use in ischemic stroke and do not make recommendations in ICH (22); there are no studies in children. A recent study analyzed data from the Cerebral Hematoma And NXY Treatment trial for neuroprotection in adults with ICH and found that prophylactic anticonvulsant use was associated with poor outcome. However, only 8% of study participants (n = 23) were placed on prophylactic medication (42). Similarly, in a prospective observational study of prophylactic anticonvulsant use in 98 adults with ICH, 12 (12%) received levetiracetam prophylaxis, 22 (22%) received phenytoin prophylaxis, 6 (6%) received both anticonvulsants, and 58 (59%) patients received neither anticonvulsant (43). In the seven patients with a clinical seizure, five occurred on the day of ICH. Phenytoin use was associated with a longer hospital stay and worse modified Rankin Scale at 14 days, 28 days, and 3 months. However, there was selection bias in these studies as patients with the largest ICH or who were sicker were most likely to receive prophylactic anticonvulsants.
Separate from the issue of preventing seizures with prophylactic anticonvulsants is the detection and management of convulsive or nonconvulsive seizures when seizures do occur. Continuous EEG monitoring is often utilized in the ICU but it is not of proven benefit to patients at this time. A recent study examined 100 children who had continuous EEG monitoring for a diverse array of clinical indications including some children with ICH and reported that EEG monitoring led to the initiation or escalation of antiseizure medications in 43 patients due to seizure detection (44). Many of these children had prolonged unresponsiveness after a seizure as the indication for EEG monitoring, so the application of these data to children with acute ICH is unclear. Continuous EEG monitoring should be strongly considered in children who have (1) persistently altered mental status that is not clearly explained by their ICH, or (2) movements or vital sign changes that are suggestive of seizure that cannot be captured on a routine EEG. In adult patients with SAH, EEG has been used to detect vasospasm (45) but this has not yet been studied in children.
■ DIAGNOSTIC STUDIES
If ICH or SAH is suspected, HCT is still considered the initial imaging study of choice by most authors (21). Magnetic resonance imaging (MRI) sequences such as gradient echo or susceptibility-weighted images clearly identify hemorrhage, but they are not universally available, may require sedation, show both old and new blood, and require some experience and training to correctly identify the hemorrhage (46). A child with ICH should have a thorough evaluation for vascular anomalies which account for 40% to 90% of pediatric ICH (3,13,14). Given this high rate of vascular malformations, high quality cerebrovascular imaging is critical. In one study of 116 children with hemorrhagic stroke between 1993 and 2003 only 65% had vascular imaging (47). A recent study documented vascular imaging in nearly 100% of nonneonates with IPH, perhaps leading to a higher percentage of confirmed ICH etiology (14). One study reported that a combination of MRI, magnetic resonance angiography (MRA), and magnetic resonance venography (MRV) images accurately identified the cause of ICH in 25 of 38 children (66%) (48). There were two MRIs that did not detect a vascular malformation that was in fact present: one patient had a mycotic aneurysm, and the other had a peripherally located AVM identified on digital subtraction angiography or conventional cerebral angiography (CCA). In the same study, CCA alone had a diagnostic yield of 61% that was statistically equivalent to the yield from the combination of MRI, MRA, and MRV (48). However, in another series of children with nontraumatic ICH, the cause of bleeding was established in 97% of children who underwent CCA compared with 80% of children who did not have angiography (3). CCA can be relatively safe, although fewer than 50% of children with ICH undergo CCA (3,49,50). Even when a vascular malformation is detected on noninvasive imaging, neurosurgeons and interventional neuroradiologists might require CCA to characterize the malformation in more detail to determine the optimal management approach. CCA can also be used to administer treatment such as embolization of an AVM or coiling of an aneurysm prior to surgery. In a recent series of children who underwent surgical resection of AVM, intraoperative angiogram was useful for identifying residual lesion so that any remaining AVM could be resected prior to incision closure (51). Other methodologies including radiosurgery that are more commonly used in adults are being evaluated in children (52).
Recently, interest in CT angiography (CTA) has increased as it can be accomplished rapidly without sedation in some children. The disadvantages compared with MRI include the ionizing radiation exposure and iodinated contrast agent exposure. However, newer CTA protocols can reduce the amount of radiation to which the child is exposed. Another difficulty with CTA is the need to time the contrast bolus in a child with a small intravenous line (53). Some unsedated children move when contrast agent is injected, degrading the study. Advantages are that CTA is more widely available, and at many centers offers excellent and possibly superior visualization of vascular structures than MRA (54). Many neurosurgeons recommend that a CTA be obtained in any child in whom the initial HCT, lumbar puncture, or clinical history suggests the presence of an aneurysmal source since it can generally be performed quickly and is noninvasive. An undiagnosed ruptured aneurysm left untreated can have significant consequences for the patient including early rebleeding (55). CTA has a sensitivity of 95% and specificity of 83% in detecting aneurysms to about 2 mm in size in the adult population (56). However, CCA remains the gold standard (57). The American Heart Association guidelines for pediatric stroke state that when no cause for ICH is found via noninvasive vascular imaging, CCA should be strongly considered (22). Additionally, if an aneurysm is suggested by noninvasive imaging, a four vessel CCA should be performed to guide treatment.
Sometimes vascular malformations are not evident, even for months after the acute hemorrhage. Therefore, when vascular imaging is normal or inconclusive in the acute setting, studies should be repeated once the clot has been reabsorbed; this often takes 2 to 8 weeks. If no vascular cause of the ICH or SAH is identified at that point, additional vascular imaging may be required even later. The timing and frequency of additional studies has not been studied, so follow-up imaging is highly individualized.
Coagulation studies and other basic laboratory tests are recommended in the American Heart Association guidelines for management of ICH (20). No studies have been done to examine the yield of an extensive evaluation for a bleeding diathesis in children with ICH. Therefore, the guidelines for the evaluation and management of stroke in infants and children simply note that an evaluation for hematologic disorders, coagulation defects, and other risk factors may be appropriate (22). However, in children in whom a primary bleeding diathesis is suspected or in children in whom vascular imaging and MRI do not identify a cause of the ICH, hematology should be consulted, and a more detailed bleeding diathesis workup may be considered (58) (Table 12.2).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


