Class
Drug
Route of administration
Timing
Mechanism of action
Antiresorptive
Denosumaba
Subcutaneously
Twice yearly
Decreases bone resorption by inhibiting RANKL
Osteoanabolic
PTH(1-34) (teriparatide)b
Subcutaneously, transdermal, chip
Daily, weekly
Increase bone turnover, with the increase in bone formation preceding and exceeding bone resorption, characterizing the “anabolic window”
PTH(1-84)c
Subcutaneously
Daily, weekly
PTHrP(1-36)
Subcutaneously
Daily
PTHrP analog
Subcutaneously
Daily
Cathepsin K inhibitors
ONO-5334
Oral
Daily
Inhibit cathepsin K, a key enzyme for bone collagen breakdown. The effect on osteoclast function is limited so that osteoclasts continue to positively signal osteoblasts. As a result, bone resorption is reduced without suppressing bone formation to an appreciable degree
Odanacatib
Oral
Weekly
Antisclerostin antibodies
Romosozumab
Intravenous and subcutaneously
Not defined yet
Inhibition of sclerostin subsequently prevents the inhibition of Wnt/β-catenin signaling. The release of inhibition in this pathway increases bone formation and reduces bone resorption
Blosozumab
Subcutaneously
Not defined yet
Nitric oxide
Isosorbide mononitrate
Oral
Daily
Direct effect on osteoclasts and osteoblasts, leading to a decrease in bone resorption and an increase in bone formation
Nitroglycerin
Transdermal
Daily
Antiresorptive Therapy
Bisphosphonates are the mainstay of osteoporotic therapy. The only recent innovation in this class is the approval of a delayed-release form of risedronate. All oral bisphosphonates require patients to take the drug in the fasting state with plain water and to wait approximately 30–60 min before eating, drinking, or taking other medications. These stipulations help to ensure maximal absorption of the bisphosphonates which are at best poorly absorbed [1]. Less than 1 % of the administered oral bisphosphonate dose is absorbed under these optimized conditions. Some individuals cannot tolerate this approach because they are unable to take drugs while fasting. Other individuals report upper gastrointestinal intolerance under these conditions. To deal with these concerns, a weekly 35 mg delayed-release form of risedronate was developed to be taken postprandially, after breakfast. The delayed-release formulation was demonstrated to be effective compared to immediate-release risedronate in a non-inferiority trial in which both groups showed similar gains in bone mineral density (BMD) at the lumbar spine and hip [2]. As with other oral bisphosphonate preparations, patients are advised to remain upright for 30 min after taking the medication to ensure successful transit of the medication.
The most recent addition to the antiresorptive class of therapeutics for osteoporosis, denosumab, is based on a mechanism that is completely different from the bisphosphonates. Receptor activator of nuclear factor-kB ligand (RANKL) is a key mediator of osteoclast formation, activity, and survival [3]. Denosumab, a fully human monoclonal antibody, binds with high affinity and specificity to RANKL and, thus, prevents its access to mature and developing osteoclasts. As a result, osteoclast-mediated bone resorption is profoundly affected. By reducing bone turnover, denosumab increases BMD [4–6]. At vertebral, hip, and other nonvertebral sites [7, 8], denosumab reduces fracture incidence, as compared to placebo controls.
The FREEDOM trial was a 3-year international, randomized, placebo-controlled trial that enrolled 7,868 postmenopausal women with osteoporosis. Subjects were randomized to receive either 60 mg of denosumab or placebo subcutaneously (SC) every 6 months [7]. Compared to placebo, subjects taking denosumab had a significant reduction in the risk of new radiographic vertebral fractures by 68 % (relative risk (RR), 0.32; 95 % confidence interval (CI), 0.26–0.41; p < 0.001), hip fracture by 40 % (RR, 0.60; 95 % CI, 0.37–0.97; p = 0.04), and all nonvertebral fractures by 20 % (RR, 0.80; 95 % CI, 0.67–0.95; p = 0.01). By 3 years, patients in the denosumab group had a 9.2 % increase in lumbar spine BMD and a 6 % increase at the total hip. Although infections are a theoretical concern [9, 10] because RANKL is expressed in lymphocytes, the rate of serious infections was not higher with denosumab treatment. However, cellulitis and eczema were seen with greater frequency in those who took denosumab.
The gains in BMD were progressive. In the phase III trials, a linear increase was seen at all sites over the entire 3-year period [9]. Gains for as long as 8 years have been reported in the phase II trial [10]. In a subset of 99 subjects from the FREEDOM trial, quantitative computed tomography of the spine and hip was performed to estimate bone strength using finite element modeling [11]. Hip and spine strength increased for the denosumab group compared with the placebo group by 14.3 % (p < 0.0001) and 22.4 % (p < 0.0001), respectively, at 36 months.
Denosumab treatment for 36 months has also been shown to reduce the incidence of new vertebral fractures in men receiving androgen deprivation therapy for nonmetastatic prostate cancer (RR, 0.38; 95 % CI, 0.19–0.78; p = 0.006). Rates of adverse events and overall incidence of infections were similar for both the treatment and placebo groups [8].
A phase III trial compared efficacy of denosumab vs. alendronate on BMD and bone turnover markers [12]. A total of 1,189 postmenopausal women with low bone mass were randomized to receive either denosumab 60 mg every 6 months or alendronate 70 mg weekly. At 12 months, subjects in the denosumab group had a greater increase in BMD at the lumbar spine, total hip, femoral neck, and 1/3 radius. Denosumab reduced the bone formation marker procollagen type I intact N-terminal propeptide (P1NP) to a greater extent than alendronate, but the median reduction in the bone resorption marker C-terminal telopeptide (CTX) was similar for both groups. There was no difference in the overall incidence of adverse events between the two treatment groups.
The reversibility of the antiresorptive effects of denosumab was shown in a 2-year extension study of a randomized, blinded, placebo-controlled, dose-ranging trial in which patients were randomly allocated to further treatment with active drug or placebo [13]. Despite a marked suppression during the treatment period with denosumab, bone turnover markers rapidly increased after the drug was discontinued, with the most pronounced changes within the first 6 months off-therapy. The increase in bone turnover markers temporarily exceeded the control, pretreatment values. This “overshoot” was associated with a reduction in BMD. Bone turnover markers and BMD returned to values near baseline after 24 months off-treatment. Subjects in whom treatment was discontinued for 12 months before denosumab was reintroduced for an additional 12 months showed increments in BMD to an extent similar to that observed during the first period of denosumab treatment.
Denosumab is approved by the FDA for the following indications: treatment of postmenopausal women with osteoporosis who are at high risk for fracture; treatment to increase bone mass in men with osteoporosis at high risk for fracture; treatment to increase bone mass in women at high risk for fracture receiving adjuvant aromatase inhibitor therapy for breast cancer; and treatment to increase bone mass in men at high risk for fracture receiving androgen deprivation therapy for nonmetastatic prostate cancer. Denosumab is also approved to prevent skeletal-related events in patients with bone metastasis from solid tumors. In Europe, denosumab is approved for the treatment of osteoporosis in postmenopausal women at increased risk for fracture and for bone loss due to hormone ablation in men with prostate cancer.
Osteoanabolic Therapy
Parathyroid hormone (1-84) [PTH(1-84)] and its fully active, foreshortened variant, PTH(1-34) (teriparatide), represent the only currently available osteoanabolic therapies for osteoporosis. Teriparatide was demonstrated to be effective in the pivotal clinical trial conducted by Neer et al. [14]. It is indicated for the treatment of men and women with advanced osteoporosis at high risk for fracture. It is also approved for the treatment of glucocorticoid-induced osteoporosis. PTH(1-84) is approved in Europe, but not in the United States, for the treatment of postmenopausal osteoporosis. Both teriparatide and PTH(1-84) are administered by subcutaneous injection daily for up to a 2-year course. Teriparatide carries an FDA-instructed “black box” warning because of toxicity noted in rats, osteosarcoma [15]. PTH(1-84) also causes osteosarcoma in rats. Concerns that this rat toxicity might also be seen in human subjects have not been substantiated. Reviews of this subject have established that after 10 years of teriparatide use and 6 years of PTH(1-84) use amounting to a cumulative experience of over one million subjects and several million patient-years, the incidence of osteosarcoma is not greater than what would be expected in the general population not exposed to these PTH formulations [16, 17].
In contrast to antiresorptive therapies, osteoanabolic agents directly stimulate bone formation. PTH therapy increases BMD in the lumbar spine, a site comprising primarily cancellous bone. Increases in the hip region are more modest and PTH therapy may actually be associated with a reduction in BMD at the distal 1/3 radius, a cortical site. PTH therapy results in an initial rapid increase in bone formation markers subsequently followed by an increase in bone resorption markers. These changes in markers of bone formation are accompanied by histomorphometric observations that confirm an effect of PTH to increase processes associated with bone formation without any early evidence for bone resorption. This effect is reminiscent of bone metabolism in growing children in whom bone modeling is the dominant process. Thereafter, teriparatide leads to an increase in bone resorption giving rise to the more typical characteristics of bone metabolism in adults, namely bone remodeling. The “anabolic window” describes the period of time when PTH stimulates bone formation directly, before bone remodeling is stimulated [18]. Even after bone turnover is stimulated, there is more ongoing bone formation than bone resorption, thus maintaining the anabolic window at least for a finite period of time.
Different Timing and Delivery Systems of Teriparatide
Weekly Administration of PTH(1-84) or Teriparatide
A randomized, double-blind, placebo-controlled trial [19] examined the use of weekly teriparatide 200 IU (56.5 μg) in healthy Japanese men and postmenopausal women (65–95 years) with 1–5 prevalent vertebral fractures and low lumbar spine BMD (<80 % of young adult mean) at any site. Subjects were randomly assigned to receive a 72-week course of weekly teriparatide injection (n = 286; 13 men) or placebo (n = 286; 10 men). Compared to placebo, treatment with teriparatide increased BMD at the lumbar spine (0.3 % vs. 6.7 %), total hip (0.1 % vs. 3.1 %), and femoral neck (−0.5 % vs. 1.8 %), and reduced the relative risk of vertebral fracture by 80 % (14.5 % vs. 3.1 %) (p < 0.01 for all). In a subset of these patients [20], a single dose of teriparatide led to a transient decrease in bone formation markers with a subsequent increase over baseline levels up to 72 h later, lasting more than 7 days after administration. Markers of bone resorption transiently increased after administration and then decreased to below baseline levels from 24 h until the next injection. Computed tomographic imaging studies of a subgroup of these patients [21] showed that teriparatide treatment increased cortical thickness and area in the femoral neck, inter-trochanter, and shaft, while tending to decrease cortical perimeter and cortical volumetric BMD in the inter-trochanter (but not the femoral neck or shaft), compared to placebo. Weekly teriparatide also improved the biomechanical properties of section modulus and buckling ratio.
After the completion of the original weekly teriparatide trial [19], 465 subjects were enrolled in a follow-up study in which patients were treated for 1 year with bisphosphonates or other therapeutic regimens at the discretion of their physicians [22]. Among the 447 subjects who completed the study, 205 were in the post-teriparatide group and 242 in the post-placebo group. Approximately 45 % of subjects in the post-teriparatide group and 54 % in the post-placebo group were treated with bisphosphonates. The other regimens included selective estrogen receptor modulators, calcitonin, alfacalcidol, or no osteoporosis drugs. New vertebral fracture occurred in 3.4 % of subjects in the post-teriparatide group and 13.7 % in the post-placebo group (RR, 0.23; 95 % CI, 0.10–0.52; p < 0.05).
Outcomes from the original weekly teriparatide trial are comparable to those obtained with daily teriparatide [14], with the exception that daily teriparatide decreased cortical BMD of the femoral neck. Weekly teriparatide is now approved for use in Japan [23].
Weekly PTH(1-84) administration has also been studied [24]. A double-blind, placebo-controlled trial randomized 50 postmenopausal women 45–70 years of age with low BMD at the femoral neck to receive PTH(1-84) 100 μg or placebo daily for 1 month, followed by weekly injections of PTH or placebo for 11 months. At 1 year, lumbar spine BMD increased by 2.1 % in PTH-treated women, significantly greater than placebo (p = 0.03), although there were no significant differences at the hip [25].
Transdermal Teriparatide
In phase I trials, a transdermal teriparatide delivery system was shown to deliver PTH(1-34) with a rapid time to maximal concentration, comparable area under the curve, and shorter half-life than the subcutaneous route [26]. A subsequent 6-month, randomized, placebo-controlled, positive control, multidose phase II trial compared a daily transdermal microneedle teriparatide patch with a placebo patch and subcutaneous teriparatide 20 μg injection in 165 postmenopausal women [27]. Bone turnover markers (P1NP and CTX) increased in all treatment groups in a dose-dependent manner compared to placebo. At 6 months, lumbar spine BMD increased by 3.0 %, 3.5 %, and 5.0 % in the 20-, 30-, and 40-μg teriparatide patch groups, respectively, and by 3.6 % in the subcutaneous teriparatide group (p < 0.001 vs. placebo (−0.3 %) for all). The 40 μg teriparatide patch increased total hip BMD compared to both placebo patch and subcutaneous teriparatide injection (p < 0.05). The 40 μg dose is entering into phase III trials [28].
Delivery of Teriparatide by Chip Technology
A novel approach to deliver teriparatide through a wirelessly controlled implantable microchip was recently described [29]. The microchip-based devices, containing discrete doses of lyophilized teriparatide, were implanted in the subcutaneous tissue of the abdomen of eight osteoporotic postmenopausal women for 4 months and wirelessly adjusted to release doses once daily for up to 3 weeks. A computer-based programmer communicated wirelessly with the implant to program the dosing schedule and to receive implant status verifying proper operation. For comparison, each study subject subsequently received subcutaneous injections of teriparatide in escalating doses. The device produced similar pharmacokinetics of teriparatide to standard daily subcutaneous injections with a lower coefficient of variation in seven of the eight study subjects. This novel approach to deliver teriparatide increased the bone formation marker P1NP, confirming that the device was clinically effective. There were no toxic or adverse events due to the device or to the drug, and patients stated that the device did not compromise quality of life. This new cutting edge technology has promise.
PTH-Related Peptides (PTHrP(1-36); PTHrP Analog)
PTH-related peptide (PTHrP) was first brought to medical attention as a cause of humoral hypercalcemia of malignancy. It was subsequently demonstrated to have key physiological actions in the skeleton as well as other systems [30]. Despite the fact that both PTH and PTHrP act at a common receptor, the proteins are products of different genes. While there is limited overall sequence homology between the two peptides, the N-terminal regions are intensely homologous. This N-terminal sequence homology helps to explain the shared interaction with a common PTH–PTHrP receptor. In the normal state, PTHrP has evolved to regulate local tissue functions, as opposed to the systemic hormonal role of PTH [31]. Similar to PTH in primary hyperparathyroidism, PTHrP is catabolic to bone when administered continuously, and therefore intermittent administration has been studied. Intermittent administration of PTHrP increases bone mass in rodents with varying potency in relationship to PTH [32].
Initial short-term studies with PTHrP(1-36) by Horwitz et al. [33, 34] showed early effects that appeared to favor a rather exclusive stimulation of bone formation. Results from a phase II study [35] in 105 postmenopausal women aged 45–75 years (n = 35 in each group) are now available. Subjects were randomized to daily treatment with PTHrP(1-36) 400 μg, PTHrP(1-36) 600 μg, or PTH(1-34) 20 μg for 3 months. The primary outcome measures were bone turnover markers, with secondary outcome measures of BMD and safety. PTH(1-34) and PTHrP(1-36) stimulated bone formation early (day 15), although by day 90 PTH(1-34) increased bone formation markers two to fourfold greater than PTH(1-36) at the 600 or 400 μg doses, respectively (p < 0.05). As expected, the increase in bone resorption occurred later (day 60 for PTH(1-34) and day 90 for both PTH(1-36) groups), and was not as dynamic. The increase in bone resorption at day 90 was threefold greater for the PTH(1-34) arm than either of the PTH(1-36) dosage groups (p < 0.05), which were not different from each other. At 3 months, PTH(1-34) and PTHrP(1-36) at both doses significantly increased BMD by about 2 % at the lumbar spine. There were small but significant increases in hip BMD in the PTHrP(1-36) groups but not in the PTH(1-34) group. There were no significant differences in BMD at the forearm. Adverse effects were similar between the PTH(1-34) and PTHrP(1-36) groups, with the exception of more frequent episodes of mild hypercalcemia in the PTHrP(1-36) group.
A PTHrP analog is being developed by Radius Health, Inc. [36]. The first 22 residues are identical to PTHrP but thereafter, from amino acids 22 to 34, the molecule contains several replacement residues designed to optimize its osteoanabolic potential. The results of a multicenter, randomized, double-blind, placebo-controlled phase II investigation of safety and effects on BMD and bone markers were recently presented [36]. Postmenopausal women with osteoporosis, aged 55–85 years, were randomized to placebo, BA058 20, 40, or 80 μg, or teriparatide 20 μg for 24 weeks. After completion of the 24-week study, subjects were then eligible to participate in a 24-week extension study. One hundred and eighty-four of 221 patients completed 6 months of treatment. The mean percent change in lumbar spine BMD at 24 weeks was 1.6 % with placebo, 2.9 %, 5.2 %, and 6.7 % with BA058 20 μg, 40 μg, and 80 μg, respectively, and 5.5 % with teriparatide (p < 0.001 compared to placebo for BA058 40 and 80 μg and teriparatide). Further dose-dependent increases in lumbar spine BMD were noted during the extension (n = 55), with a mean percent change at 48 weeks of 0.7 % with placebo, 5.1 %, 9.8 %, and 12.9 % with BA058 20 μg, 40 μg, and 80 μg, respectively, and 8.6 % with teriparatide. The investigators also found dose-dependent increases in total hip BMD. At 24 weeks, changes were noted in serum and urine bone turnover markers from baseline (p ≤ 0.05) for BA058 40 and 80 μg and for teriparatide. BA058 was well tolerated, with an adverse event profile comparable with blinded placebo. BA058 is the focus of a phase III placebo-controlled, 18-month international study in postmenopausal women [24]. The primary endpoint is the incidence of new vertebral fractures at a dose of 80 μg in comparison to placebo. A transdermal microneedle technology is also under development for BA058 and in phase I and II trials (www.radiuspharm.com).
Combination Osteoanabolic and Antiresorptive Therapy
In theory, the combination of an antiresorptive and osteoanabolic agent offers the potential for increased efficacy over monotherapy with either drug class given their differing mechanisms of action. If bone resorption is inhibited by an antiresorptive while bone formation is stimulated by an osteoanabolic agent, combination therapy might give better results than therapy with either agent alone. Despite the intuitive attraction of this reasoning, important data to the contrary have been provided by Black et al. [25] and Finkelstein et al. [37]. These two groups independently conducted trials using a form of PTH alone, alendronate alone, or the combination of the PTH formulation and alendronate. Black et al. studied postmenopausal women treated with 100 μg of PTH(1-84) and Finkelstein et al. studied men given 40 μg of teriparatide. PTH monotherapy was associated with greater densitometric gains than with combination therapy or alendronate alone at the lumbar spine with both dual energy X-ray absorptiometry (DXA) and quantitative computed tomography. Combination therapy was not different from alendronate alone. Bone turnover markers followed the expected course for monotherapy with anabolic or antiresorptive agents. For combination therapy, however, bone markers followed the course of alendronate, not PTH, with reductions in bone formation and bone resorption markers. The findings from these two trials suggest that the impaired response to combination therapy was due to the dominating effects of alendronate on bone remodeling dynamics when both drugs are used in combination.
The results of these combination therapy studies with alendronate led to the concept that an antiresorptive agent that did not impair the anabolic actions of PTH to increase bone formation while mitigating its effects on bone resorption may be a more effective approach to combination therapy. Deal et al. [38] studied the effects of raloxifene, a less potent antiresorptive agent than the bisphosphonates, in combination with teriparatide over 6 months. Their results support the idea that combination therapy might be advantageous with a mild antiresorptive drug but are not conclusive because of the short duration of the study. As a further test of this hypothesis, Walker et al. [39] investigated the combination of teriparatide and risedronate, a bisphosphonate with less potent effects on bone turnover than alendronate or zoledronic acid. Men with low BMD were randomized to receive risedronate 35 mg weekly plus daily injected placebo, teriparatide 20 μg subcutaneously daily plus weekly oral placebo, or risedronate plus teriparatide (combination) for 18 months. At study conclusion, all three treatment arms significantly increased lumbar spine BMD, the primary endpoint, but there were no between-group differences. In contrast, at the total hip, BMD increased to a greater extent in the combination group (3.86 ± 9.2 %) vs. teriparatide (0.29 ± 8.0 %) or risedronate alone (0.82 ± 8.0 %; p < 0.05 for both). Bone turnover markers in the combination group paralleled the teriparatide alone arm, supporting the idea that an antiresorptive that does not have a profound effect on bone turnover might permit salutary effects of combination therapy with an osteoanabolic agent. The results of this proof-of-concept study are favorable but require further investigation.
Cosman et al. [40] studied the use of a single dose of zoledronic acid in combination with daily teriparatide. This approach was based upon animal studies of Gasser et al. [41] in which a single dose of zoledronic acid led to greater improvements in BMD in rats treated simultaneously with teriparatide than those treated with bisphosphonate therapy alone. With the combination of zoledronic acid and teriparatide, BMD increased after 6 months at the spine and hip to a greater degree than in either monotherapy arm. With combination therapy, there was a rapid, but only transient, reduction in bone turnover markers. At the lumbar spine, there were significantly greater changes after 6 months with combination therapy but by the end of the study at 12 months, there were no differences between combination therapy and teriparatide alone (7.3 % vs. 7.3 %; p = NS). At the hip, similar results were observed with the combination therapy arm showing a greater enhancement of BMD than zoledronic acid alone, but by the 12-month endpoint of the study, differences were no longer appreciated (2.3 % vs. 2.2 %; p = NS). However, if one considered the lumbar spine and hip sites together as a composite endpoint, only combination therapy provided improvement in BMD that was greater than either zoledronic acid or teriparatide alone.
The first section of this chapter is devoted to denosumab, a potent inhibitor of RANKL. Several interesting properties have surfaced over continued investigation of this therapy. The distal 1/3 radius, a cortical site, increases and remains above baseline for 8 years. The second observation is that PTH levels rise after denosumab administration by approximately twofold and remain above baseline for about 3 of the 6-month interval between doses [5, 42, 43]. To relate these observations to each other requires appreciation of the fact that PTH requires RANKL for its catabolic actions [44–46]. As a RANKL inhibitor, denosumab blocks this catabolic pathway. The increase in PTH associated with denosumab, therefore, may preferentially exploit the anabolic Wnt signaling pathway [47, 48]. The increase in cortical bone density (distal 1/3 radius) is compatible with this hypothesis. Seeman et al. [49] have provided additional evidence for this view when they showed that the higher PTH in connection with denosumab is associated with lower cortical porosity. In the control arm of this study, alendronate administration was associated with increases in PTH but there was a positive relationship with higher PTH levels associated with greater porosity. These observations led to the hypothesis that denosumab and teriparatide in combination may be more beneficial than the combination of teriparatide with other antiresorptives. The Denosumab and Teriparatide Administration (DATA) Study [50] has in fact shown a densitometric benefit to combination therapy in postmenopausal women. At 12 months, lumbar spine BMD increased more in the denosumab and teriparatide combination group (n = 30; 9.1 %) than the teriparatide alone group (n = 31; 6.2 %, p = 0.014) or denosumab alone (n = 33; 5.5 %, p = 0.0005). Femoral neck BMD also increased to a greater extent in the combination group (4.2 %) than the teriparatide alone (0.8 %, p = 0.0007) or denosumab alone arms (2.1 %, p = 0.0238), with similar findings at the total hip site. There was an increase of 2.6 % in BMD at the distal 1/3 radius in the combination therapy arm and 1.7 % in the denosumab alone arm without between-group differences, although both groups differed when compared to teriparatide alone, which demonstrated a 1.8 % decline in BMD. As expected, teriparatide alone increased bone turnover markers and denosumab alone decreased bone turnover markers. While the combination therapy group decreased the bone resorption marker CTX to a similar extent as the denosumab alone group, bone formation decreased more gradually and to a lesser extent.
It should be noted that these and other combination studies have not been designed with fracture outcome as a definitive endpoint. These trials have only targeted surrogate endpoints such as BMD and bone turnover markers, and have also been shorter than typical definitive fracture trials. One must therefore exercise caution in the interpretation of these data. In addition, the use of two pharmacologic therapies simultaneously carries with it the potential for more adverse events than the use of a single therapy as well as added expense.
Newer Therapies
Cathepsin K Inhibitors
Cathepsin K, a member of the papain family of cysteine proteases, is highly expressed by activated osteoclasts, and promotes degradation of type I collagen (Fig. 12.1) [51]. It plays an important role in the process of bone resorption through its actions to remove collagen, an initial step in the creation of the bone remodeling unit [51]. Clues to the therapeutic potential of inhibiting the actions of cathepsin K came from studies of a human genetic disorder, pycnodysostosis, a disorder of excess bone due to an inactivating point mutation in the gene encoding cathepsin K [52, 53]. Several oral compounds have been designed to inhibit cathepsin K [54]. Preclinical studies of cathepsin K inhibitors noted that the drug reduces bone resorption without suppressing bone formation to an appreciable degree [55–57]. Different from the bisphosphonates, cathepsin K inhibitors appear to permit certain functions of the osteoclast such as signaling to the osteoblast while preventing its classical actions to excavate bone.
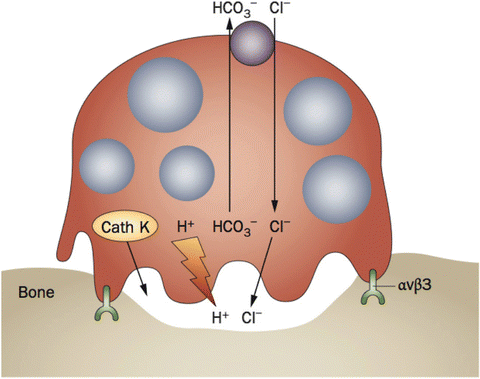

Fig. 12.1




Cartoon depicting osteoclast-mediated bone resorption. Hydrochloric acid and proteases, including cathepsin K (Cath K), are secreted into the sealing zone created by the adhesion of the osteoclast to bone through integrin αvβ3. The organic collagen matrix, 90 % of which is composed of type I collagen, is subsequently degraded. Reprinted by permission from Macmillan Publishers Ltd: Costa AG, Cusano NE, Silva BC, Cremers S, Bilezikian JP. Cathepsin K: its skeletal actions and role as a therapeutic target in osteoporosis. Nat Rev Rheumatol. 2011;7(8):447-456
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


