FIGURE 22.1 Nonenhanced head computed tomography (CT) obtained because of poor drainage from the ventriculostomy. The proximal catheter is suboptimally placed. Patient was taken back to the operating room for repositioning and returned with adequate cerebrospinal fluid drainage.
Unfortunately, some patients do leave the OR without a working ventriculostomy, do not get a CT scan, and arrive in the PICU with a malfunctioning ventriculostomy. The burden then falls to the PICU team. There are many reasons why a ventriculostomy would not drain CSF, but an externalized ventriculostomy that is not draining CSF should be attributed to a malpositioned catheter until proven otherwise. The differential diagnosis of a nonfunctioning ventriculostomy includes (1) a malpositioned catheter, (2) stopcocks accidentally turned to the off position, (3) catheter clogged with blood, brain, or air, (4) no CSF in the brain, (5) very low pressure in the brain, or (6) a disconnected or kinked catheter. All of these potential etiologies can be easily assessed. First, confirm that all of the stopcocks are in the open position and there are no kinks. Second, inspect the tubing for debris blocking CSF flow. Third, drop the ventriculostomy bag to the floor to see if lowering the pressure results in drainage. These maneuvers can all be done in minutes. If there is still no CSF drainage, obtain a head CT. If the catheter is in good position and there is CSF in the ventricular system, the treatment is gentle flushing of the system. If the catheter is still not draining after flushing, then it may need to be replaced. Even when the orders from the neurosurgical team are to keep the catheter clamped, periodically checking the ventriculostomy is required to ensure that there is a good waveform and that dropping the bag several centimeters results in CSF flow. If these maneuvers do not result in either a good waveform or egress of CSF, then the neurosurgical team should be notified.
Lumbar Drains
Lumbar drains are most commonly placed in pediatric patients to stop CSF leaks after anterior skull base surgery or trauma, and in certain spine procedures. Lumbar drains are not benign and can cause a myriad of complications. Complications begin at the time of insertion and can continue for several days after they are removed.
Lumbar drains are placed by inserting a Touhy needle (14 gauge) into the lumbar cistern and threading the catheter into the lumbar cistern. Patients can complain of radicular pain from injury during insertion or of severe back and leg pain every time the drain is opened. Lumbar drains are difficult to secure and can easily be accidentally dislodged. Low pressure headaches and infections are also not uncommon.
The most severe complication is tension pneumocephalus (Figure 22.2). The typical presentation is a patient who underwent an anterior skull base procedure and has lumbar drain placed to divert the CSF and allow the skull base repair to heal to a watertight seal. Air entering the nose is now in communication with the intracranial cavity and, because of the CSF flow out through the lumbar drain, a downward herniation of the brain can occur causing headache, obtundation, CN deficits, and even death. The signs and symptoms need to be recognized early and treated immediately. The treatment is clamping of the lumbar drain followed by removal of the drain with emergent placement of a blood patch (3). It is critical to recognize that this condition can develop and worsen even if the drain is clamped, or within 24 to 48 hours after the drain is removed. The treatment in this case is emergent blood patch.
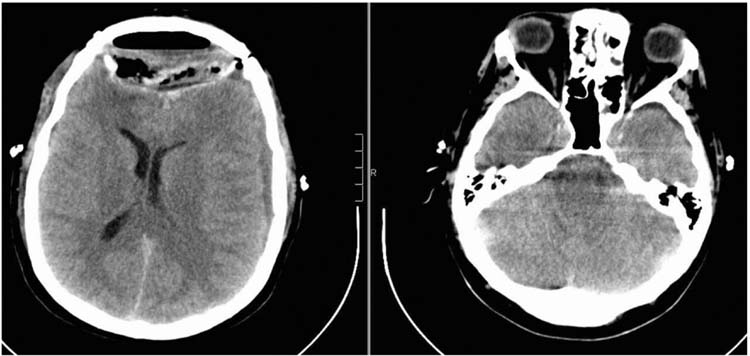
FIGURE 22.2 Nonenhanced head CT obtained because the patient became increasingly confused 24 hours after an anterior skull base procedure. The patient’s lumbar drain was clamped; however, he continued to deteriorate. The image shows classic signs of low pressure changes. Patient improved hours after getting an emergent blood patch.
Posterior Fossa Brain Tumors
Approximately 50% of pediatric brain tumors are in the posterior fossa. The three most common tumors are medulloblastoma, juvenile pilocytic astrocytoma, and ependymoma. The most common presentation is secondary to the obstructive hydrocephalus which develops when these tumors block CSF flow through the fourth ventricle. Children present with headaches, nausea, and vomiting. Head CT or magnetic resonance imaging (MRI) will demonstrate a posterior fossa mass (Figure 22.3A and B). These tumors often need urgent (24–72 hours) treatment to relieve the pressure. In the absence of a hemorrhage into the tumor causing an acute increase of the hydrocephalus, these patients rarely need an emergent ventriculostomy.
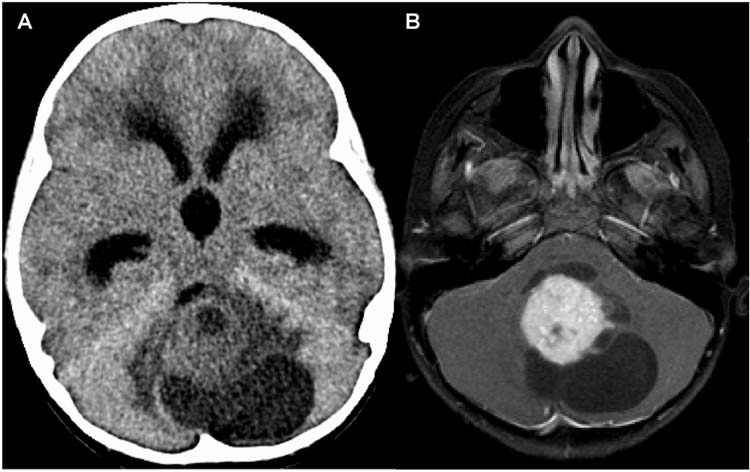
FIGURE 22.3 (A) Nonenhanced head CT showing obstructive hydrocephalus and a posterior fossa mass. (B) Axial magnetic resonance imaging (MRI) after gadolinium administration in the same patient. Tumor is originating in the cerebellum, strongly enhancing with multiple cysts. Patient underwent resection and the diagnosis was juvenile pilocytic astrocytoma.
Typically, these tumors are treated by placing an externalized ventriculostomy with or without an endoscopic third ventriculostomy. This is generally safe but, as discussed above, there are important postoperative considerations with externalized ventriculostomies. Next the patient undergoes a midline incision, the bone is removed (we advocate a craniotomy, but some surgeons do a craniectomy), and the dura opened. The vermis is divided to provide better visualization, but it is important to limit manipulation of the vermis because of the postoperative complications of cerebellar mutism (also known as posterior fossa syndrome) (4,5). The tumor is then carefully removed with continuous inspection of the floor of the fourth ventricle. It is not uncommon for these tumors to become adherent to the floor of the fourth ventricle, especially in the case of juvenile pilocytic astrocytomas. These tumors can sometimes originate from the floor of the fourth where they are exophytic brainstem tumors filling the fourth ventricle (Figure 22.4). It is important for the PICU team and the neurosurgeon to discuss the extent of vermian and fourth ventricular floor involvement.
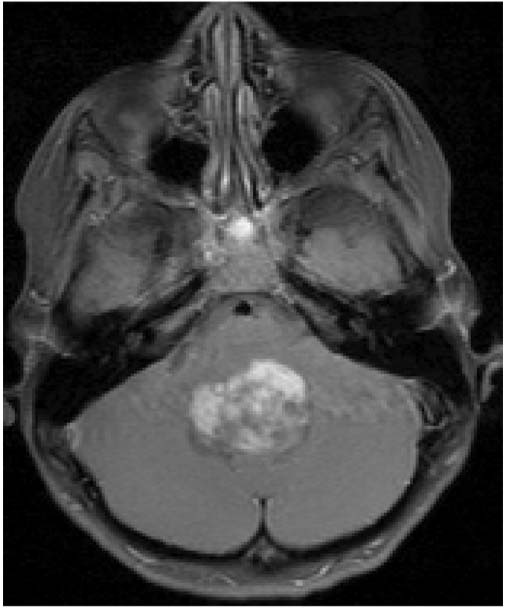
FIGURE 22.4 Axial MRI after gadolinium administration. The tumor is originating from the floor of the fourth ventricle. The tumor was unable to be completely resected, and the patient had transient intraoperative and postoperative hemodynamic instability.
Splitting the vermis, especially the superior vermis in patients between the ages of 7 and 11 years of age who have a medulloblastoma, increases the risk of postoperative mutism. When severe, patients may not be able to be extubated, will be unable to speak or be hypophonic (speaking softly as if whispering), will have tremors and titubations, and will have severe strabismus and abnormal ocular movements. It is important to distinguish abnormal ocular movement abnormalities related to vermian involvement from those reflecting CN involvement, especially CN 6, but also CN 3 and CN 4. If the floor of the fourth ventricle is involved, then the patient is also at risk for CN 7 injury because of the dorsal location of the facial colliculus. Patients with injury to the facial colliculus will have a peripheral CN 7 palsy and may also have a CN 6 palsy. The presence of both CN 6 and CN 7 paresis or palsies localizes the injury to the brainstem, in contrast to the presence of both CN 7 and CN 8 injuries which localizes the lesion to the cerebellopontine angle.
Another concern with tumors invading the brainstem is possible postoperative hemodynamic instability if the nuclei of CN 9 and 10 are involved (6). Usually there are episodes of severe bradycardia and hypertension intraoperatively when this occurs, so the PICU team should carefully inspect the anesthesia record and ask the neurosurgeon about the extent of brainstem involvement and possible hemodynamic changes in the OR. It is unlikely that the nuclei of CN 12 would be injured but this may occur with inferior medullary exophytic tumors. In the unlikely event of bilateral CN 12 injuries the tongue will fall back into the pharynx and lead to severe airway obstruction. If this deficit is permanent, then tracheostomy is required.
The most common postoperative complications of a midline posterior fossa tumor are hemorrhage and hydrocephalus. Understanding the tumor location and the postoperative examination is important in evaluating any changes in the postoperative period. For example, if the patient had a medulloblastoma that was not adherent to the floor of the fourth ventricle and several hours after surgery the patient became lethargic with hemodynamic instability, pinpoint pupils (pontine lesion), diplopia or sluggish pupils, then this is a hemorrhage until proven otherwise and emergent head CT is needed. A less likely explanation of these postoperative findings is a stroke, but that is extremely uncommon and the deficit would likely present immediately and be detected in the OR or in the PICU’s initial examination. Our policy is that if the patient is unable to be extubated or has any unexpected change in exam immediately after extubation in the OR, then he or she goes directly for a head CT. A postoperative CT is key in identifying hemorrhage, poor ventriculostomy placement, hydrocephalus, pneumocephalus, and stroke. While head CT is likely to miss small strokes, it would be unlikely to miss any complication severe enough to produce an examination change. The other complication to consider is related to the reaccumulation of hydrocephalus. The signs and symptoms are the same as discussed above.
Some posterior fossa tumors extend out the foramen of Luschka and grow into the cerebellopontine angle. This is almost exclusively a feature of ependymomas, but can also occur in atypical teratoid rhabdoid tumors or medulloblastoma.
Patients with neurofibromatosis type 2 have bilateral seventh nerve schwannomas. Patients like these with tumors in the cerebellopontine angle are at risk for postoperative injuries to CNs 7, 8, 9, 10, 11, and 12 (Figure 22.5). Resection is performed with concurrent neuromonitoring of these nerves, but patients are at risk for postoperative aspiration even when the nerves stimulate normally at the end of the case. It is better to leave the endotracheal tube in for an extra day or two than to extubate prematurely and risk an aspiration or respiratory arrest. These patients require speech and swallowing evaluation prior to initiating oral nutrition.
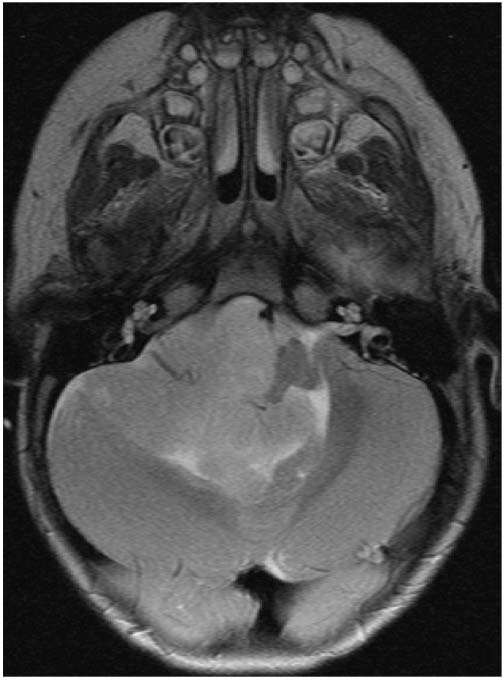
FIGURE 22.5 MRI axial T2-weighted image at the level of cranial nerves 7 and 8 showing a very large fourth ventricular ependymoma growing out into the right cerebellopontine angle, putting the patient at risk for postoperative lower cranial nerve dysfunction.
Suprasellar Tumors
The two most common pediatric tumors in the sellar and suprasellar region are hypothalamic gliomas (with or without a neurofibromatosis type 1 diagnosis) and craniopharyngiomas. Hypothalamic tumors should be suspected in children under the age of 4 with tumors that do not have calcium on a head CT and are not cystic. Younger children often carry the diagnosis of failure to thrive (Russell syndrome) and may have nystagmus. Patients with neurofibromatosis type 1 and a hypothalamic glioma have a better prognosis than patients without neurofibromatosis type 1. Many patients with hypothalamic gliomas do not need surgery and are managed by the neurooncologists alone. However, if the tumors are very large and causing mass effect on the brainstem, obstructive hydrocephalus, or have radiographic features that are atypical or concerning for a more aggressive tumor, then they will be debulked. Because these tumors grow out of the optic nerves and chiasm, they cannot be completely removed surgically.
The postoperative complications here are ischemia related to vasospasm of the anterior and middle cerebral arteries and/or seizures related to hyponatremia. Children younger than 2 years of age are at greatest risk (7,8). If vasospasm is encountered in the OR, then papaverine is placed on the affected artery and the patient is managed by elevating the postoperative mean arterial pressure (MAP) and aggressive fluid goals. We usually aim for a MAP of 10% higher than the patient’s baseline MAP during the start of the procedure. We also consider the calcium channel blocker nimodipine, but have to carefully weigh the risks and benefits in a young child because nimodipine administration may lower MAPs. Intraoperative vasospasm is usually related to direct manipulation of the arteries. Postoperative vasospasm is often a result of arteries that were under severe stretch from a large tumor which develop vasospasm as they relax (Figure 22.6). These patients require frequent monitoring for signs and symptoms of vasospasm. The typical presentation is a waxing and waning neurologic exam that is exquisitely sensitive to blood pressure. In this situation, it is imperative to keep the patient in positive fluid balance and keep the blood pressure elevated with pressors. The duration of vasospasm for this patient population is not as well known. We typically monitor closely for 48 hours, and if there are no signs of vasospasm on exam or postresection MRI/A, then we relax the MAP goals and slowly wean fluids, while closely monitoring for subtle neurologic changes. If the patient is symptomatic, then we continue close monitoring, aggressive fluid goals, and an elevated MAP for 48 hours beyond the last symptoms, and then we slowly relax these strategies. However, there is no good literature on this patient population.
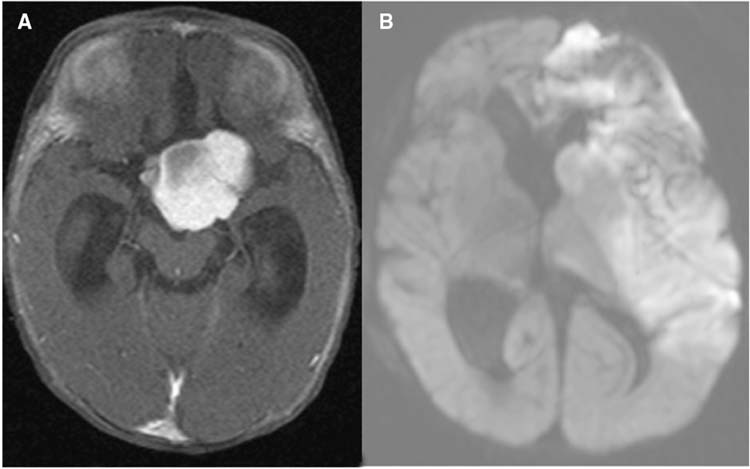
FIGURE 22.6 Preoperative (A) and postoperative (B) MRI of a chiasmatic hypothalamic glioma. (A) Axial MRI after gadolinium administration showing a large enhancing suprasellar tumor. (B) Diffusion weighted MRI showing a large left-sided stroke that occurred on postoperative day 4 from vasospasm.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


