Primary
Tension-type (69%)
Migraine (16%)
Cluster and other trigeminal autonomic cephalgias (< 1%)
Other
Secondary
Systemic infection (63%)
Head or neck trauma (4%)
Cranial or cervical vascular disorders
Intracranial nonvascular
Substance use or withdrawal
Disorders of homeostasis
Head and neck disorders
Psychiatric disorders
 Tension-Type Headache
Tension-Type Headache
These are most common, and characteristic features include muscle tightness and mild to moderate pain that can persist for hours in the back of the neck and head. There are no associated neurological disturbances or nausea. The pain usually responds to rest, massage, application of heat or ice, antiinflammatory medications, or mild tranquilizers.
 Migraine Headache
Migraine Headache
These headaches have a 1-year prevalence in all women of approximately 15 percent, and thus they are frequently encountered during pregnancy. The term migraine describes a periodic, sometimes incapacitating neurological disorder characterized by episodic attacks of severe headache and autonomic nervous system dysfunction (Goadsby, 2012). The International Headache Society (2004) classifies three migraine types based on the presence or absence of an aura as well as chronicity:
1. Migraine without aura—was formerly termed common migraine—and is characterized by a unilateral throbbing headache, nausea and vomiting, or photophobia.
2. Migraine with aura—formerly termed classic migraine—has similar symptoms preceded by premonitory neurological phenomena such as visual scotoma or hallucinations. A third of patients have this type of migraine, which sometimes can be averted if medication is taken at the first premonitory sign.
3. Chronic migraine is defined by a migraine headache occurring at least 15 days each month for more than 3 months.
Migraines may begin in childhood, peak in adolescence, and tend to diminish in both frequency and severity with advancing years. According to Lipton and associates (2007), their annual prevalence is 17 percent in women and 6 percent in men. Another 5 percent of women have probable migraine, that is, they have all criteria but one (Silberstein, 2007). Migraines are especially common in young women and have been linked to hormone levels (Torelli, 2010).
The exact pathophysiology of migraines is uncertain, but they occur when neuronal dysfunction leads to decreased cortical blood flow, activation of vascular and meningeal nociceptors, and stimulation of trigeminal sensory neurons (Brandes, 2007; D’Andrea, 2010). A predilection for the posterior circulation has been described by Kruit and coworkers (2004). Migraines—especially those with aura in young women—are associated with increased risk for ischemic strokes as discussed on page 1191. The risk is greater in those who smoke or use combination oral contraceptives.
Migraine in Pregnancy
The prevalence of migraine headaches in the first trimester is 2 percent (Chen, 1994). Prospective as well as observational studies have shown that most migraineurs have improvement during pregnancy (Adeney, 2006; Menon, 2008; Torelli, 2010). Still, migraines—usually those with an aura—occasionally appear for the first time during pregnancy. Pregnant women with preexisting migraine symptoms may have other symptoms suggestive of a more serious disorder, and new neurological symptoms should prompt a complete evaluation (Detsky, 2006; Heaney, 2010).
Although conventional thinking has been that migraine headaches do not pose increased maternal or fetal risks, several recent studies have refuted this (Allais, 2010). For example, women with severe migraines in the first 8 weeks may be at slightly increased risk for a fetus with limb-reduction defects (Banhidy, 2006). Preeclampsia and other cardiovascular morbidities are also increased (Facchinetti, 2009; Sanchez, 2008; Schürks, 2009). In a case-control study of nearly 18.5 million pregnancy-related discharges from 2000 through 2003, Bushnell and coworkers (2009) identified an incidence of migraine discharge codes of 185 per 100,000. Associated diagnoses and increased significant risks were found for migraines and stroke—15.8-fold; myocardial infarction—4.9; heart disease—2.1; venous thromboembolism—2.4; and preeclampsia/gestational hypertension—2.3-fold.
Management
Data are limited regarding nonpharmacological management in pregnancy such as biofeedback techniques, acupuncture, and transcranial magnetic stimulation (Airola, 2010; Dodick, 2010). Effective pharmacological interventions include nonsteroidal antiinflammatory drugs, and most migraine headaches respond to simple analgesics such as ibuprofen or acetaminophen, especially if given early. Because of patient idiosyncrasies, multitarget drug therapy is necessary in most cases for migraine relief (Gonzalez-Hernandez, 2014). Severe headaches should be treated aggressively with intravenous hydration and parenteral antiemetics and opioids. Although intravenous magnesium sulfate has gained favor in the past few years, a recent metaanalysis reported no beneficial effects (Choi, 2014). Ergotamine derivatives are potent vasoconstrictors that should be avoided in pregnancy (Briggs, 2011).
Triptans are serotonin 5-HT1B/2D-receptor agonists that effectively relieve headaches by causing intracranial vasoconstriction (Contag, 2010). They also relieve nausea and vomiting and greatly reduce the need for analgesics. They can be given orally, by injection, as a rectal suppository, or as a nasal spray. The greatest experience is with sumatriptan (Imitrex), and although not studied extensively in pregnancy, it appears to be safe (Briggs, 2011; Nezvalová-Henriksen, 2010).
For women with frequent migraine headaches, oral prophylactic therapy is warranted. Amitriptyline (Elavil), 10 to 150 mg daily; propranolol (Inderal), 20 to 80 mg three times daily; or metoprolol (Lopressor, Toprol), 50 to 100 mg twice daily, is safe in pregnancy and has been used with success (Contag, 2010; Lucas, 2009; Marcus, 2007).
 Cluster Headaches
Cluster Headaches
This rare primary headache disorder is characterized by severe unilateral lancinating pain radiating to the face and orbit, lasting 15 to 180 minutes, and occurring with autonomic symptoms and agitation. Pregnancy does not affect symptom severity. Affected women should avoid tobacco and alcohol. Acute management includes 100-percent oxygen therapy and sumatriptan given as a 6-mg dose subcutaneously (Calhoun, 2010; Francis, 2010). If recurrent, prophylaxis is administered using a calcium-channel blocking agent.
SEIZURE DISORDERS
The Centers for Disease Control and Prevention reported that the prevalence of epilepsy in adults in 2005 was 1.65 percent (Kobau, 2008). There are 1.1 million American women of childbearing age who are affected. After headaches, seizures are the next most prevalent neurological condition encountered in pregnant women, and they complicate 1 in 200 pregnancies (Brodie, 1996; Yerby, 1994). Importantly, epilepsy accounted for 13 percent of maternal deaths in the United Kingdom for the 2005 to 2007 triennium (Lewis, 2007). Seizure disorders are also associated with altered fetal development, and they can adversely affect other pregnancy outcomes. The teratogenic effects of several anticonvulsant medications are unquestioned. The American Academy of Neurology and the American Epilepsy Society have developed guidelines regarding treatment in pregnant women, which are discussed subsequently (Harden, 2009a–c).
 Pathophysiology
Pathophysiology
A seizure is defined as a paroxysmal disorder of the central nervous system characterized by an abnormal neuronal discharge with or without loss of consciousness. Epilepsy encompasses different syndromes whose cardinal feature is a predisposition to recurrent unprovoked seizures. The International League Against Epilepsy Commission on Classification and Terminology recently updated its terminologies for seizures (Berg, 2010a, 2011; Shorvon, 2011). This new classification schema is under development, and for now, most adults can be said to have either focal or generalized seizures.
Focal Seizures
These originate in one localized brain area and affect a correspondingly localized area of neurological function. They are believed to result from trauma, abscess, tumor, or perinatal factors, although a specific lesion is rarely demonstrated. Focal seizures without dyscognitive features start in one region of the body and progress toward other ipsilateral areas of the body, producing tonic and then clonic movements. Simple seizures can affect sensory function or produce autonomic dysfunction or psychological changes. Cognitive function is not impaired, and recovery is rapid. Focal seizures with dyscognitive features are often preceded by an aura and followed by impaired awareness manifested by sudden behavioral arrest or motionless stare. Involuntary movements such as picking motions or lip smacking are common.
Generalized Seizures
These involve both brain hemispheres and may be preceded by an aura before an abrupt loss of consciousness. There is a strong hereditary component. In generalized tonic-clonic seizures, loss of consciousness is followed by tonic contraction of the muscles and rigid posturing, and then by clonic contractions of all extremities while the muscles gradually relax. Return to consciousness is gradual, and the patient may remain confused and disoriented for several hours. Absence seizures—also called petit mal seizures—are a form of generalized epilepsy that involve a brief loss of consciousness without muscle activity and are characterized by immediate recovery of consciousness and orientation.
 Causes of Seizure
Causes of Seizure
Some identifiable causes of convulsive disorders in young adults include head trauma, alcohol- and other drug-induced withdrawals, cerebral infections, brain tumors, biochemical abnormalities, and arteriovenous malformations. A search for these is prudent with a new-onset seizure disorder in a pregnant woman. The diagnosis of idiopathic epilepsy is one of exclusion.
 Preconceptional Counseling
Preconceptional Counseling
Women with epilepsy should undergo education and counseling before pregnancy (Chap. 8, p. 158). Folic acid supplementation with 0.4 mg per day is begun at least 1 month before conception. The dose is increased to 4 mg when the woman taking antiepileptic medication becomes pregnant. These medications are assessed and adjusted with a goal of monotherapy using the least teratogenic medication. If this is not feasible, then attempts are made to reduce the number of medications used and to use them at the lowest effective dose (Dunlop, 2008). Medication withdrawal should be considered if a woman is seizure free for 2 years or more.
 Epilepsy During Pregnancy
Epilepsy During Pregnancy
The major pregnancy-related risks to women with epilepsy are increased seizure rates with attendant mortality risks and fetal malformations. Seizure control is the main priority. Earlier studies described worsening seizure activity during pregnancy, however, this is not as common nowadays because of more effective drugs. Contemporary studies cite increased seizure activity in only 20 to 30 percent of pregnant women (Mawer, 2010; Vajda, 2008; Viinikainen, 2006). Women who are seizure free for at least 9 months before conception will likely remain so during pregnancy (Harden, 2009b).
Increased seizure frequency is often associated with decreased and thus subtherapeutic anticonvulsant serum levels, a lower seizure threshold, or both. An impressive number of pregnancy-associated alterations can result in subtherapeutic serum levels. These include nausea and vomiting, decreased gastrointestinal motility, antacid use that diminishes drug absorption, pregnancy hypervolemia offset by protein binding, induction of hepatic enzymes such as cytochrome oxidases, placental enzymes that metabolize drugs, and increased glomerular filtration that hastens drug clearance. Importantly, some women discontinue medication because of teratogenicity concerns. Finally, the seizure threshold can be affected by pregnancy-related sleep deprivation as well as hyperventilation and pain during labor.
Pregnancy Complications
Women with epilepsy have a small increased risk of some pregnancy complications (Borthen, 2011; Harden, 2009b). A population-based study from Iceland found that epileptic women had a twofold increased cesarean delivery rate (Olafsson, 1998). In a cohort study from Montreal, Richmond and coworkers (2004) reported an increased incidence of nonproteinuric hypertension and labor induction. From a Swedish study of 1207 epileptic women, Pilo and colleagues (2006) reported a 1.5-fold increased incidence of cesarean delivery, preeclampsia, and postpartum hemorrhage. Postpartum depression rates have also been reported to be increased in epileptic women (Turner, 2009). Finally, children of epileptic mothers have a 10-percent risk of developing a seizure disorder.
Embryo-Fetal Malformations
For years, it was difficult to separate effects of epilepsy versus its therapy as the primary cause of fetal malformations. As discussed in Chapter 8 (p. 158), it is now believed that untreated epilepsy is not associated with an increased fetal malformation rate (Thomas, 2008; Viinikainen, 2006). That said, the fetus of an epileptic mother who takes certain anticonvulsant medications has an indisputably increased risk for congenital malformations. Moreover, monotherapy is associated with a lower birth defect rate compared with multiagent therapy. Thus, if necessary, increasing monotherapy dosage is at least initially preferable to adding another agent (Buhimschi, 2009).
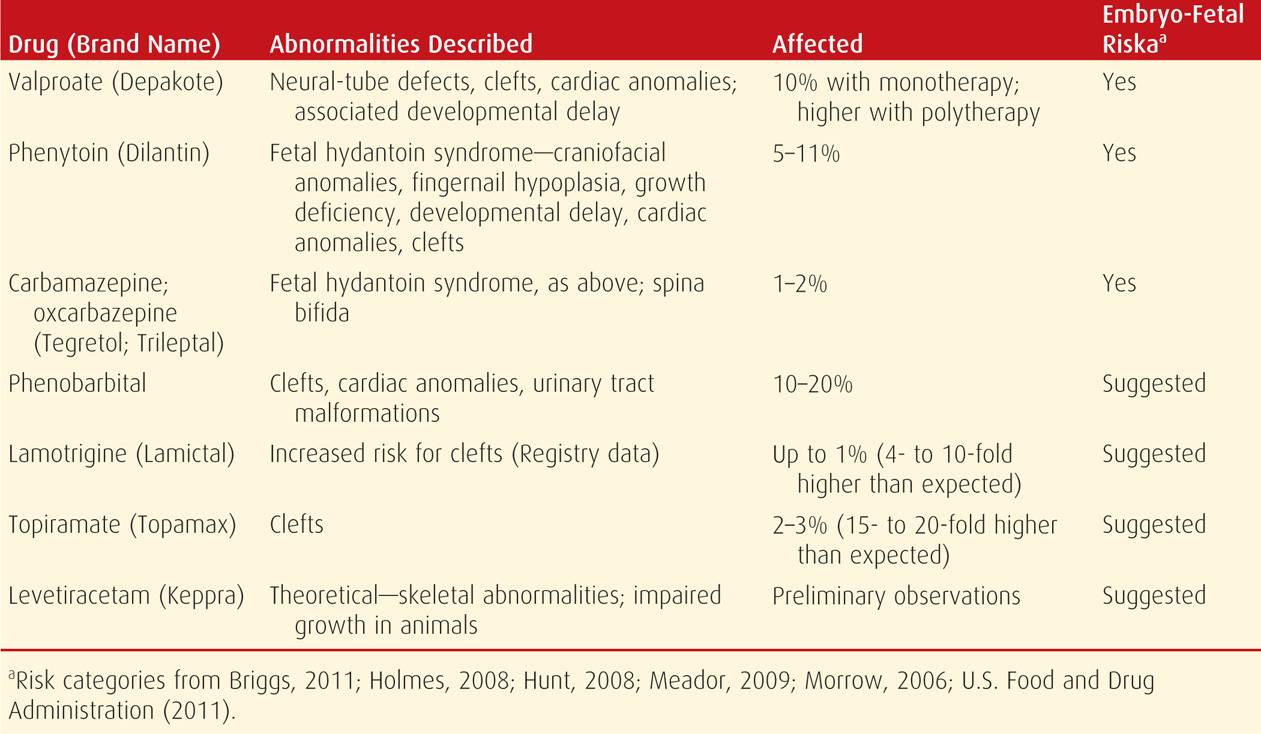
Specific drugs, when given alone, increase the malformation rate (Chap. 12, p. 246). Some of these are listed in Table 60-2. Phenytoin and phenobarbital increase the major malformation rate two- to threefold above baseline (Perucca, 2005; Thomas, 2008). A particularly potent teratogen is valproate, which has a dose-dependent effect and increases the malformation risk four- to eightfold (Eadie, 2008; Klein, 2014; Wyszynski, 2005). In general, with polytherapy, the risk increases with each drug added. At least at this time, the newer antiepileptic medications are reported to have no associations with a markedly increased risk of major birth defects (Molgaard-Nielson, 2011).
TABLE 60-2. Teratogenic Effects of Common Anticonvulsant Medications
Management in Pregnancy
The major goal is seizure prevention. To accomplish this, treatment for nausea and vomiting is provided, seizure-provoking stimuli are avoided, and medication compliance is emphasized. The fewest necessary anticonvulsants are given at the lowest dosage effective for seizure control. Although some providers routinely monitor serum drug levels during pregnancy, these concentrations may be unreliable because of altered protein binding. Free or unbound drug levels, although perhaps more accurate, are not widely available. Importantly, there is no evidence that such monitoring improves seizure control (Adab, 2006). For these reasons, drug levels may be informative if measured following seizures or if noncompliance is suspected. Some of the newer agents such as lamotrigine and oxcarbazepine may be more amenable to serum drug level monitoring (Harden, 2009a; Pennell, 2008).
For women taking anticonvulsant drugs, a targeted sonographic examination at midpregnancy is recommended by some to search for anomalies (Chap. 10, p. 197). Testing to assess fetal well-being is generally not indicated for women with uncomplicated epilepsy.
Breast Feeding and Contraception
There are limited available data regarding the safety of breast feeding with the various anticonvulsant medications. That said, no obvious deleterious effects, such as long-term cognitive issues, have been reported (Briggs, 2011; Harden, 2009c). Some of the anticonvulsant agents are associated with increased oral contraceptive failures. Thus, other more reliable methods should be considered (Chap 38, p. 696).
CEREBROVASCULAR DISEASES
Abnormalities of the cerebrovascular circulation include strokes—both ischemic and hemorrhagic, as well as anatomical anomalies, such as arteriovenous malformations and aneurysms. The current endemic of obesity in this country, along with concomitant increases in heart disease, hypertension, and diabetes, has also resulted in increased stroke prevalence (Centers for Disease Control and Prevention, 2012). Women have a higher lifetime risk of stroke than men as well as higher associated mortality rates (Martínez-Sánchez, 2011; Roger, 2012). Moreover, pregnancy increases the immediate and lifetime risk of both ischemic and hemorrhagic stroke (Jamieson, 2010; Jung, 2010).
Stroke is relatively uncommon in pregnant women, but it contributes disparately to maternal mortality rates. Reported incidences of strokes in pregnancy range from 1.5 to 71 per 100,000 pregnancies (James, 2005; Kuklina, 2011; Scott, 2012). The incidence is increasing as measured by pregnancy-related hospitalizations for stroke (Callaghan, 2008; Kuklina, 2011). Importantly, most are associated with hypertensive disorders or heart disease. Almost 9 percent of the pregnancy-related mortality rate in the United States is due to cerebrovascular accidents, with a third being associated with preeclampsia (Berg, 2010b).
 Risk Factors
Risk Factors
Most strokes in pregnancy manifest either during labor and delivery or in the puerperium. In a study of 2850 pregnancy-related strokes, approximately 10 percent developed antepartum, 40 percent intrapartum, and almost 50 percent postpartum (James, 2005). Several risk factors—unrelated and related to pregnancy—have been reported from studies that included more than 10 million pregnancies. These include older age; migraines, hypertension, obesity, and diabetes; cardiac disorders such as endocarditis, valvular prostheses, and patent foramen ovale; and smoking. Those related to pregnancy include hypertensive disorders, gestational diabetes, obstetrical hemorrhage, and cesarean delivery. By far, the most common risk factors are pregnancy-associated hypertensive disorders. As noted, one third of strokes are associated with gestational hypertension, and there is a three- to eightfold increased risk of stroke in hypertensive compared with normotensive women (Scott, 2012; Wang, 2011). Women with preeclampsia undergoing general anesthesia may be at higher risk of stroke compared with those given neuraxial anesthesia (Huang, 2010). Another risk factor for peripartum stroke is cesarean delivery, which increases the risk 1.5-fold compared with vaginal delivery (Lin, 2008).
Pregnancy-induced effects on cerebrovascular hemodynamics are unclear as related to risk for stroke. Although cerebral blood flow decreased by 20 percent from midpregnancy until term, importantly, it increased significantly with gestational hypertension (Zeeman, 2003, 2004b). Such hyperperfusion would at least intuitively be dangerous in women with certain vascular anomalies.
 Ischemic Stroke
Ischemic Stroke
Acute occlusion or embolization of an intracranial blood vessel causes cerebral ischemia, which may result in death of brain tissue (Fig. 60-1). The more common associated conditions and etiologies of ischemic stroke are shown in Table 60-3. A transient ischemic attack (TIA) is caused by reversible ischemia, and symptoms usually last less than 24 hours. Patients with a stroke usually have a sudden onset of severe headache, hemiplegia or other neurological deficits, or occasionally, seizures. Focal neurological symptoms accompanied by an aura usually signify a first-episode migraine (Liberman, 2008).
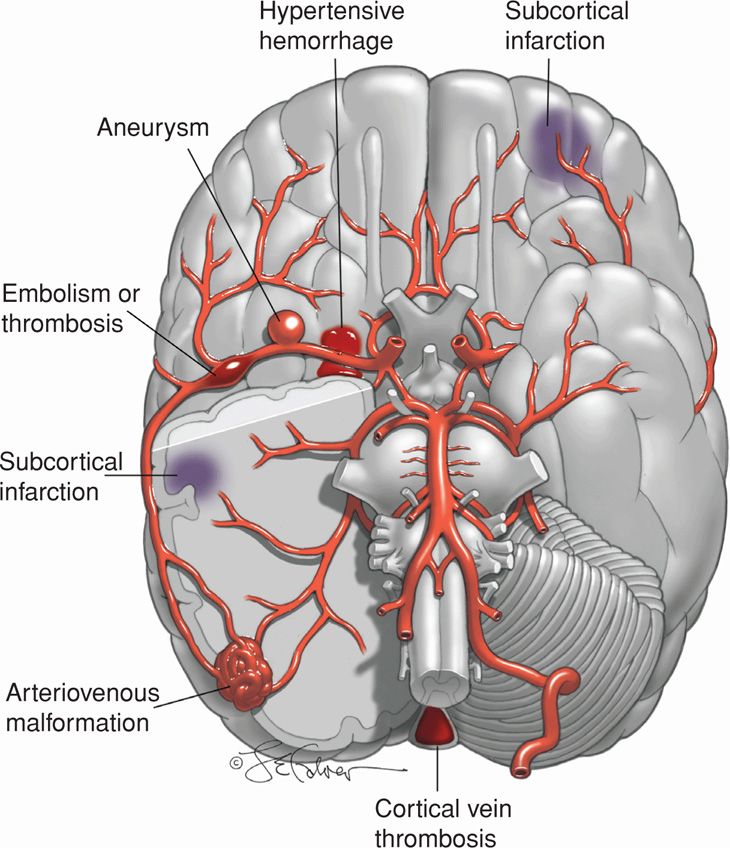
FIGURE 60-1 Illustrations of a brain showing various types of strokes seen in pregnancy: (1) subcortical infarction (preeclampsia), (2) hypertensive hemorrhage, (3) aneurysm, (4) embolism or thrombosis in middle cerebral artery, (5) arteriovenous malformation, and (6) cortical vein thrombosis.
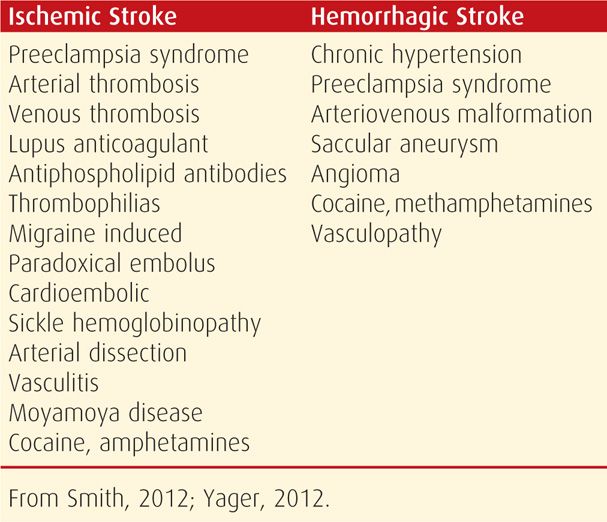
TABLE 60-3. Some Associated Disorders or Causes of Ischemic and Hemorrhagic Strokes During Pregnancy or the Puerperium
Evaluation of an ischemic stroke includes echocardiography and cranial imaging with CT, MR, or angiography. Serum lipids are measured with the caveat that their values are distorted by normal pregnancy (Appendix, p. 1291). Antiphospholipid antibodies and lupus anticoagulant are sought—these cause up to a third of ischemic strokes in otherwise healthy young women (Chap. 59, p. 1173). Also, tests for sickle-cell syndromes are completed when indicated. With a thorough evaluation, most causes can be identified, although treatment is not always available. Some of these include cardiac-associated embolism, vasculitis, or vasculopathy such as Moyamoya disease (Ishimori, 2006; Miyakoshi, 2009; Simolke, 1991).
Preeclampsia Syndrome
In reproductive-age women, a significant proportion of pregnancy-related ischemic strokes are caused by gestational hypertension and preeclampsia syndrome (Jeng, 2004). As shown in Figure 60-1, areas of subcortical perivascular edema and petechial hemorrhage may progress to cerebral infarction (Aukes, 2007, 2009; Zeeman, 2004a). Although these are usually clinically manifest by an eclamptic convulsion, a few women will suffer a symptomatic stroke from a larger cortical infarction (Chap. 40, p. 742).
Other conditions with findings similar to preeclampsia include thrombotic microangiopathies (Chap. 56, p. 1116) and the reversible cerebral vasoconstriction syndrome (Chap. 40, p. 743). The latter, which is also termed postpartum angiopathy, can cause extensive cerebral edema with necrosis as well as widespread infarction with areas of hemorrhage (Katz, 2014; Ramnarayan, 2009; Singhal, 2009).
Cerebral Embolism
These strokes usually involve the middle cerebral artery (see Fig. 60-1). They are more common during the latter half of pregnancy or early puerperium (Lynch, 2001). The diagnosis can be made with confidence only after thrombosis and hemorrhage have been excluded. The diagnosis is more certain if an embolic source is identified. Hemorrhage may be more difficult to exclude because embolization and thrombosis are both followed by hemorrhagic infarction. Paradoxical embolism is an uncommon cause, even considering that more than a fourth of adults have a patent foramen ovale through which right-sided venous thromboemboli are deported (Kizer, 2005; Scott, 2012). Foraminal closure may not improve outcomes in these patients, however, this procedure has been performed during pregnancy (Dark, 2011; Furlan, 2012). Assorted cardioembolic causes of stroke include arrhythmias—especially atrial fibrillation, valvular lesions, mitral valve prolapse, mural thrombus, infective endocarditis, and peripartum cardiomyopathy.
Management of embolic stroke in pregnancy consists of supportive measures and antiplatelet therapy. Thrombolytic therapy and anticoagulation are controversial issues at this time (Kizer, 2005; Li, 2012).
Cerebral Artery Thrombosis
Most thrombotic strokes affect older individuals and are caused by atherosclerosis, especially of the internal carotid artery. Many are preceded by one or more transient ischemic attacks. Thrombolytic therapy with recombinant tissue plasminogen activator—rt-PA or alteplase is recommended within the first 3-hour window if there is measurable neurological deficit and if neuroimaging has excluded hemorrhage. This can be used in pregnancy. A principal risk is hemorrhagic transformation of an ischemic stroke in approximately 5 percent of treated patients (van der Worp, 2007).
Cerebral Venous Thrombosis
In a 10-center study in the United States, 7 percent of cerebral venous thromboses were associated with pregnancy (Wasay, 2008). Even so, pregnancy-associated cerebral venous thrombosis is rare in developed countries, and reported incidences range from 1 in 11,000 to 1 in 45,000 pregnancies (Lanska, 1997; Simolke, 1991). In the Nationwide Inpatient Sample of more than 8 million deliveries, James and associates (2005) observed that venous thrombosis caused only 2 percent of pregnancy-related strokes (Saposnik, 2011). There are numerous predisposing causes, and the greatest risk is in late pregnancy and the puerperium.
Thrombosis of the lateral or superior sagittal venous sinus usually occurs in the puerperium and often in association with preeclampsia, sepsis, or thrombophilia (see Fig. 60-1). It is more common in patients with inherited thrombophilias, lupus anticoagulant, or antiphospholipid antibodies (Chaps. 52, p. 1029 and 59, p. 1173). Headache is the most common presenting symptom, neurological deficits are common, and up to a third of patients have convulsions (Wasay, 2008). Diagnosis is with MR venography (Saposnik, 2011).
Management includes anticonvulsants for seizures, and although heparinization is recommended by most, its efficacy is controversial (de Freitas, 2008; Saposnik, 2011; Smith, 2012). Antimicrobials are given if there is septic thrombophlebitis, and fibrinolytic therapy is reserved for those women failing systemic anticoagulation. The prognosis for venous thrombosis in pregnancy is better than in nonpregnant subjects, and mortality rates are less than 10 percent (McCaulley, 2011). The recurrence rate is 1 to 2 percent during a subsequent pregnancy (Mehraein, 2003).
Recurrence Risk of Ischemic Stroke
Women with previous ischemic stroke have a low risk for recurrence during a subsequent pregnancy unless a specific, persistent cause is identified. Lamy and colleagues (2000) followed 37 women who had an ischemic stroke during pregnancy or the puerperium, and none of their 24 subsequent pregnancies was complicated by another stroke. In another study of 23 women who had prepregnancy strokes from a variety of causes, there were 35 subsequent pregnancies without a stroke recurrence (Coppage, 2004). Finally, in a follow-up study of 1770 nonpregnant women with antiphospholipid-related ischemic stroke, investigators reported no difference in the recurrence risk as long as preventative treatment was given with warfarin or aspirin (Levine, 2004). In another study of nonpregnant subjects, low-dose aspirin following venous thromboembolism decreased the risk of a subsequent stroke (Brighton, 2012).
Currently, there are no firm guidelines regarding prophylaxis in pregnant women with a stroke history (Helms, 2009). The American Heart Association stresses the importance of controlling risk factors such as hypertension and diabetes (Furie, 2011). Women with antiphospholipid antibody syndrome or certain cardiac conditions should be considered for prophylactic anticoagulation as discussed in Chapters 49 (p. 979) and 59 (p. 1175).
 Hemorrhagic Stroke
Hemorrhagic Stroke
The two distinct categories of spontaneous intracranial bleeding are intracerebral and subarachnoid hemorrhage. The symptoms of a hemorrhagic stroke are similar to those of an ischemic stroke. Their differentiation is only possible with CT or MR imaging (Morgenstern, 2010).
Intracerebral Hemorrhage
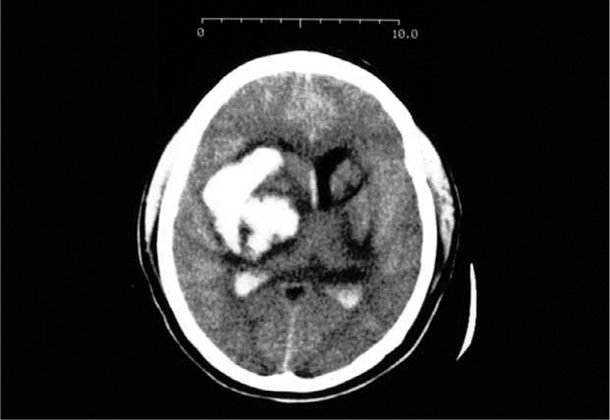
Bleeding into the brain parenchyma most commonly is caused by spontaneous rupture of small vessels previously damaged by chronic hypertension as depicted in Figure 60-1 (Qureshi, 2001; Takebayashi, 1983). Thus, pregnancy-associated hemorrhagic strokes such as the one shown in Figure 60-2 are often associated with chronic hypertension and superimposed preeclampsia (Cunningham, 2005; Martin, 2005). Because of its location, this type of hemorrhage has much higher morbidity and mortality rates than does subarachnoid hemorrhage (Morgenstern, 2010). Chronic hypertension is uniquely associated with Charcot-Bouchard microaneurysms of the penetrating branches of the middle cerebral artery. Pressure-induced rupture causes bleeding in the putamen, thalamus, adjacent white matter, pons, and cerebellum. In the 28 women described by Martin and associates (2005), half died and most survivors had permanent disabilities. This cautions for the importance of proper management for gestational hypertension—especially systolic hypertension—to prevent cerebrovascular pathology (Chap. 40, p. 761).
Subarachnoid Hemorrhage
In a study of 639 cases of pregnancy-related subarachnoid hemorrhage from the Nationwide Inpatient Sample, the incidence was 5.8 per 100,000 pregnancies, with half being postpartum (Bateman, 2012). These bleeds are more likely caused by an underlying cerebrovascular malformation in an otherwise normal patient (see Fig. 60-1). Ruptured saccular or “berry” aneurysms cause 80 percent of all subarachnoid hemorrhages. The remaining cases are caused by a ruptured arteriovenous malformation, coagulopathy, angiopathy, venous thrombosis, infection, drug abuse, tumors, or trauma. Such cases are uncommon, and a ruptured aneurysm or angioma or bleeding from a vascular malformation has an incidence of 1 in 75,000 pregnancies. Although this frequency is not different from that in the general population, the mortality rate during pregnancy is reported to be as high as 35 percent.
Intracranial Aneurysm. Approximately 2 to 5 percent of adults have this lesion (see Fig. 60-1). Fortunately, only a small percentage rupture—approximately 0.1 percent for aneurysms < 10 mm and 1 percent for those > 10 mm (Smith, 2008). Most aneurysms identified during pregnancy arise from the circle of Willis, and 20 percent are multiple (Stoodley, 1998). Pregnancy does not increase the risk for aneurysmal rupture. However, because of their high prevalence, they are more likely to cause subarachnoid bleeding than other causes (Hirsch, 2009; Tiel Groenestege, 2009). Aneurysms are more likely to bleed during the second half of pregnancy—only approximately 20 percent bleed during the first half (Dias, 1990).
The cardinal symptom of a subarachnoid hemorrhage from an aneurysm rupture is sudden severe headache, accompanied by visual changes, cranial nerve abnormalities, focal neurological deficits, and altered consciousness. Patients typically have signs of meningeal irritation, nausea and vomiting, tachycardia, transient hypertension, low-grade fever, leukocytosis, and proteinuria. Prompt diagnosis and treatment may prevent potentially lethal complications. The American Heart Association recommends noncontrast cranial CT imaging as the first diagnostic test, although MR imaging may be superior (Chalela, 2007; Connolly, 2012).
Treatment includes bed rest, analgesia, and sedation, with neurological monitoring and strict blood pressure control. Repair of a potentially accessible aneurysm during pregnancy depends in part on the recurrent hemorrhage risk versus surgical risks. At least in nonpregnant patients, the risk of subsequent bleeding with conservative treatment is 20 to 30 percent for the first month and then 3 percent per year. The risk of rebleeding is highest within the first 24 hours, and recurrent hemorrhage leads to death in 70 percent.
Early repair is done by surgical clipping of the aneurysm or by endovascular coil placement completed using fluoroscopic angiography yet attempting to limit radiation exposure. For women remote from term, repair without hypotensive anesthesia seems optimal. For women near term, cesarean delivery followed by aneurysm repair is a consideration, and we have successfully done this in several cases. For aneurysms repaired either before or during pregnancy, most allow vaginal delivery if remote from aneurysmal repair. A problem is what defines “remote,” and although some recommend 2 months, the time for complete healing is unknown. For women who survive subarachnoid hemorrhage, but in whom surgical repair is not done, we agree with Cartlidge (2000) and recommend against bearing down—put another way, we favor cesarean delivery.
Arteriovenous Malformations. These are congenital focal abnormal conglomerations of dilated arteries and veins with subarteriolar disorganization (see Fig. 60-1). They lack capillaries and have resultant arteriovenous shunting. Although unclear, the risk of bleeding may increase with gestational age. When arteriovenous malformations (AVMs) bleed, half do so into the subarachnoid space, whereas half are intraparenchymal with subarachnoid extension (Smith, 2008). They are uncommon and are estimated to occur in 0.01 percent of the general population. Bleeding does not appear to be more likely during pregnancy (Finnerty, 1999; Horton, 1990). AVMs are correspondingly rare during pregnancy, and in the study from Parkland Hospital, there was only one AVM in nearly 90,000 deliveries (Simolke, 1991).
Treatment of AVMs in nonpregnant patients is largely individualized. There is no consensus whether all those that are accessible should be resected. It also depends on whether the lesion is symptomatic or an incidental finding; its anatomy and size; presence of associated aneurysm, which is found in up to 60 percent; and especially, whether or not the lesion has bled. After hemorrhage, the risk of recurrent bleeding in unrepaired lesions is 6 to 20 percent within the first year, and 2 to 4 percent per year thereafter (Friedlander, 2007; Smith, 2008). The mortality rate with a bleeding AVM is 10 to 20 percent. In pregnancy, the decision to operate is usually based on neurosurgical considerations, and Friedlander (2007) recommends strong consideration for treatment if bleeding occurs. Because of the high risk of recurrent hemorrhage from an unresected or inoperable lesion, we favor cesarean delivery. An unusual case of spontaneous regression of a cerebral AVM has been described (Couldwell, 2011).
DEMYELINATING OR DEGENERATIVE DISEASES
The demyelinating diseases are neurological disorders characterized by immune-mediated focal or patchy destruction of myelin sheaths accompanied by an inflammatory response. The degenerative diseases are multifactorial and are characterized by progressive neuronal death.
 Multiple Sclerosis
Multiple Sclerosis
In the United States, multiple sclerosis (MS) is second only to trauma as a cause of neurological disability in middle adulthood (Hauser, 2012b). Because the disease affects women twice as often as men and usually begins in the 20s and 30s, women of reproductive age are most susceptible. The familial recurrence rate of MS is 15 percent, and the incidence in offspring is increased 15-fold.
The demyelinating characteristic of this disorder results from predominately T cell-mediated autoimmune destruction of oligodendrocytes that synthesize myelin. There is a genetic susceptibility and likely an environmental trigger such as exposure to certain bacteria and viruses, for example, Chlamydophila pneumoniae, human herpesvirus 6, or Epstein-Barr virus (Frohman, 2006; Goodin, 2009).
There are four clinical types of multiple sclerosis (Hauser, 2012b):
1. Relapsing-remitting MS accounts for initial presentation in 85 percent of affected individuals. It is characterized by unpredictable recurrent episodes of focal or multifocal neurological dysfunction usually followed by full recovery. Over time, however, relapses lead to persistent deficits.
2. Secondary progressive MS disease is relapsing-remitting disease that begins to pursue a progressive downhill course after each relapse. It is likely that all patients eventually develop this type.
3. Primary progressive MS accounts for 15 percent of cases. It is characterized by gradual progression of disability from the time of initial diagnosis.
4. Progressive-relapsing MS refers to primary progressive MS with apparent relapses.
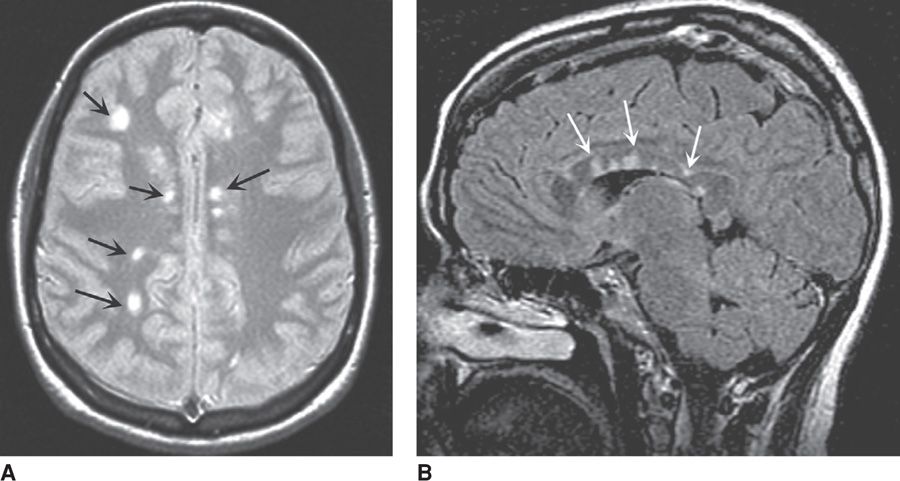
Classic findings of MS include sensory loss, visual symptoms from optic neuritis, weakness, paresthesias, and a host of other neurological symptoms. Almost 75 percent of women with isolated optic neuritis develop multiple sclerosis within 15 years. Clinical diagnosis is confirmed by MR imaging and cerebrospinal fluid analysis. In greater than 95 percent of cases, MR imaging shows characteristic multifocal white matter plaques that represent discrete areas of demyelination such as shown in Figure 60-3. Their appearance and extent are less helpful for treatment response. Similarly, Kuhle and colleagues (2007) reported that identification of serum antibodies against myelin oligodendrocyte glycoprotein and myelin basic protein are not predictive of recurrent disease activity.
FIGURE 60-3 Magnetic resonance cranial images from a woman with multiple sclerosis. A. T2-weighted axial image shows bright signal abnormalities in white matter, typical for multiple sclerosis. B. Sagittal T2-FLAIR image shows hyperintense areas within the corpus callosum that are representative of demyelination in multiple sclerosis. (From Hauser, 2012b, with permission.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree