2 Neonatology
General Techniques of Physical Examination
Assessment of the Newborn
The purposes of the routine newborn assessment are to determine the infant’s gestational age, document normal growth and development for a given gestational age, uncover signs of birth-related trauma or congenital anomalies, and evaluate the overall health and condition of the infant. The assessment begins with the establishment of a historical database. Information may be obtained from antenatal, labor, delivery, and postpartum records and a brief interview with the parents (Fig. 2-1). The aim of this data gathering is to assess the fetal and neonatal responses to pregnancy, labor, and delivery; to estimate the risk for hereditary or congenital diseases; and to identify the potential for future difficulties by reviewing the family’s social history and observing maternal–infant interactions. This background is recorded in the infant’s medical record and serves as a guide to the subsequent physical examination (Table 2-1).
Table 2-1 Newborn Historical Database
| Antenatal Record |
| Labor and Delivery Record |
| Postpartum Record |
| Parental Interview |
Observation must be done before the quiet infant is disturbed by the examination. By visual inspection the clinician can assess skin and facies; general tonus and symmetry of movement; respiratory rate, retractions, and color; and abdominal contour. Auscultation of the heart and lungs should be done before more stressful portions of the examination, which are likely to make the infant fussy. Allowing the infant to suck on a gloved finger can help quiet the infant and permit assessment of sucking strength and palate integrity. Lifting the infant under the arms (Fig. 2-2) and gently rocking him or her (such that the head swings toward and away from the examiner) is usually calming. This maneuver also induces a reflexive opening of the eyes, which assists the ophthalmologic examination. Sucking also induces eye opening. Such maneuvers may be necessary to convince the examiner that the patient does not have a congenital cataract or an intraorbital mass (see Chapter 19) requiring prompt intervention.
Careful evaluation of the hip joints is a crucial part of each newborn examination because identification and early treatment of congenital dislocation can prevent later disability. Although asymmetry of the buttocks and skin creases or asymmetry of femoral length can be clues to dislocation, the performance of at least one of a number of active motion tests is essential. The Ortolani maneuver involves placing the third or fourth finger over the greater trochanter and the thumb on the medial aspect of the thighs (Fig. 2-3). The thighs are first adducted to try to dislocate a dislocatable hip and then abducted with the fingers pushing toward the midline and the thumbs away from midline to relocate a dislocated hip. A definite “clunk” can be felt and often heard if the femoral head has been dislocated and then moves back into the acetabulum. Often, higher-pitched clicks and snaps that represent nothing more than tendons passing over bone or cartilage can be heard and felt.
Assessment of Gestational Age
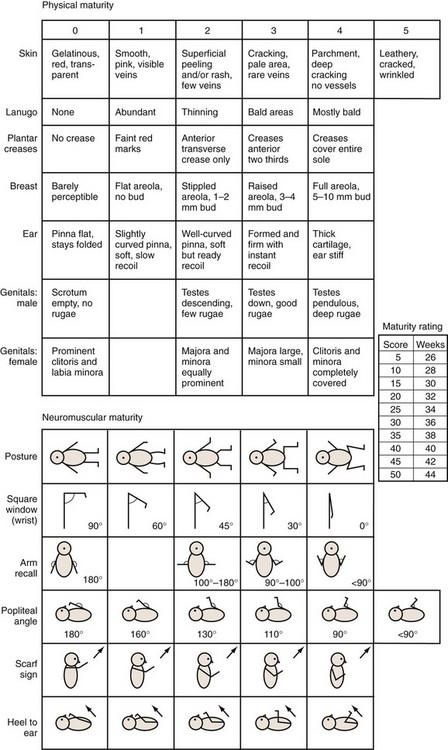
The Ballard assessment for gestational age determination of newborns uses six morphologic and six neurologic criteria to estimate gestational age on the basis of an examination performed at 12 to 24 hours of life (Fig. 2-4). Individual findings are scored on a scale of 0 to 5, and the total score is compared with the chart shown in Figure 2-4.
Physical Maturity
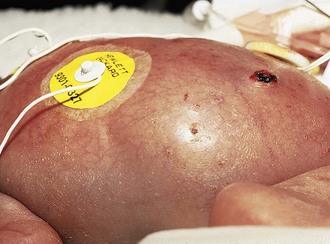
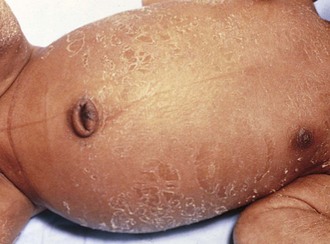
One of the most striking differences among newborns of various gestational ages is the quality of the skin. The chemical nature of skin changes during intrauterine development, with a gradual decrease in water content and a thickening of the keratin layer. Very premature infants (24 to 28 weeks) have nearly translucent, paper-thin skin (Fig. 2-5) that is easily abraded. A diffuse red hue and a prominent venous pattern are characteristic. At term, the skin no longer appears thin, and the general color is a pale pink. Some superficial peeling and cracking around the ankles and wrists may be visible. Postterm infants (42 to 44 weeks) often have more diffuse peeling and cracking of the skin because the outermost layers are sloughed (Fig. 2-6).
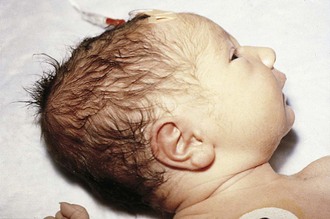
The general quality of scalp hair changes during development from rather fine, thin hair (24 to 28 weeks) to coarser, thicker hair (term). Racial differences in hair quality can make this change difficult to assess. A second type of hair, known as lanugo, appears and disappears during development. Lanugo is fine body hair that resembles peach fuzz. It is absent before weeks 20 to 22, becomes diffuse until weeks 30 to 32, and then begins to thin. Assessment of the presence and extent of lanugo is best accomplished by observing the back tangentially (Fig. 2-7).
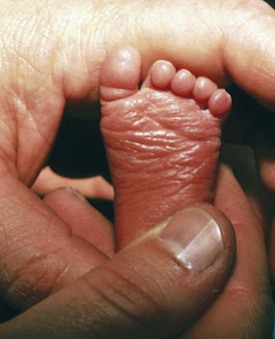
Transverse creases begin to appear on the anterior portion of the soles of the feet at approximately 32 weeks (Fig. 2-8). By 36 weeks the anterior two thirds of the sole is covered with creases. For adequate assessment of this feature, it is necessary to stretch the skin over the sole gently to distinguish wrinkling from true creases. Infants with congenital neurologic dysfunction involving the lower extremities and infants with pedal edema may lack normal creases.
Breast tissue, which is responsive to maternal hormonal influences, shows progressive increase in size as gestational age advances. Infants born at younger than 28 weeks’ gestation have barely perceptible breast tissue (see Fig. 2-5). With advancing age, breast tissue increases in size (see Fig. 2-6) and, occasionally, a term infant has active glandular secretions, which resolve spontaneously. Breast tissue can remain palpable for 2 to 3 months.
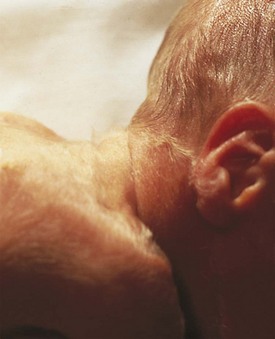
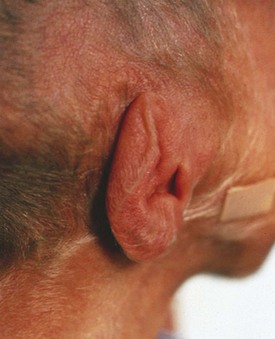
Cartilaginous development proceeds in an orderly manner during gestation and can be assessed by examination of the external ear. Although the normal incurving of the upper pinnae begins at 33 to 34 weeks and is complete at term, it is more reliable to assess the extent of cartilage in the pinnae by feeling its edge and folding the ear (Fig. 2-9). Until approximately 32 weeks, there is only minimal recoil of a folded ear, but by term there is instant recoil.
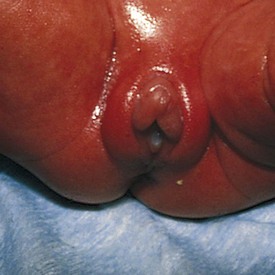
The appearance of the genitalia can be used to assess gestational age. In a boy the testes descend into the scrotum during the last month of gestation, but they are often palpable in the inguinal canal by 28 to 30 weeks. The appearance of rugae on the scrotum parallels testicular migration, appearing first on the anterior scrotum at 36 weeks and covering the entire scrotal sac by 40 weeks. Absence of testicular descent alters the appearance of the scrotum at term. Clearly, congenital cryptorchidism complicates this evaluation. In a girl the labia majora tend to be overshadowed by the clitoris and labia minora until 34 to 36 weeks (Fig. 2-10). In cases of fetal malnutrition, lack of subcutaneous fat, which should normally be present in the latter part of gestation, can interfere with assessment of the female genitalia.
Neuromuscular Maturity
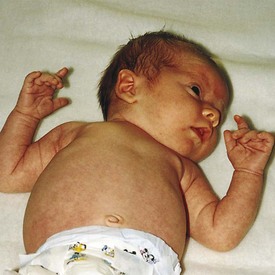
The resting supine posture of infants changes with advancing gestational age. The mature infant exhibits a marked flexor posture of the extremities compared with the extensor posture of the premature infant (Fig. 2-11).
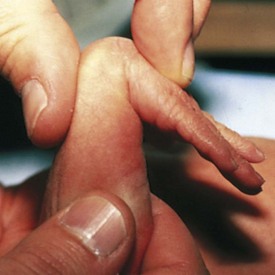
Tests for flexion angles assess a combination of muscle tone, ligament and tendon laxity, as well as flexion–extension development. The inexperienced examiner usually assumes that the very premature infant is the most flexible, but observation of flexion angles demonstrates that this is false. The square-window test of the wrist (Fig. 2-12) is performed by gently flexing the hand on the wrist and assessing the resultant angle. The wrists of babies younger than approximately 32 weeks can be flexed only to 45 to 90 degrees, whereas the wrists of term infants undergo full flexion. Sometime between birth and adulthood this flexion ability is lost. Examination of the flexion of the knees reveals a different pattern of development, with decreasing flexibility as gestational age increases. The knee is completely flexed (Fig. 2-13, A) and the thigh is stabilized against the stomach. The leg is extended by raising the foot (Fig. 2-13, B). Gentleness is essential in these evaluations because any result can be achieved if the examiner applies undue force.
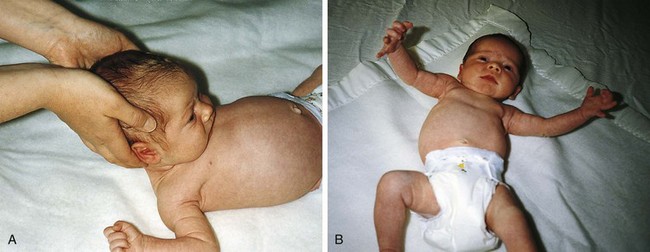
The resting tone of the upper extremities can be assessed by eliciting the scarf sign. Gentle traction of the upper extremities across the chest in a rostral direction (“placing a scarf on the infant”) while examining the position of the elbow reveals a decreasing displacement of the elbow as gestational age increases (Fig. 2-14).
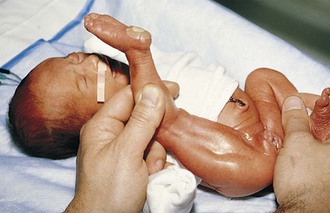
In a similar manner the resting tone of the lower extremities can be assessed by the heel-to-ear maneuver. With the baby on its back and the pelvis flat, a foot is moved as near to the ipsilateral ear as possible without exerting undue force. Very premature infants can easily touch their heels to their ears (Fig. 2-15). This becomes somewhat more difficult after 30 weeks and impossible by week 34 of gestation.
Primitive Reflexes
Normal newborns exhibit a large number of easily elicited primitive reflexes that are often altered or absent in the infant with neurologic impairment. These reflexes may be transiently depressed in the infant who has experienced difficulty in achieving the transition between intrauterine and extrauterine existence. The persistent absence or asymmetry of one or more of these reflexes may be a clue to the potential presence of neuromuscular abnormalities requiring further investigation (see Chapter 3).
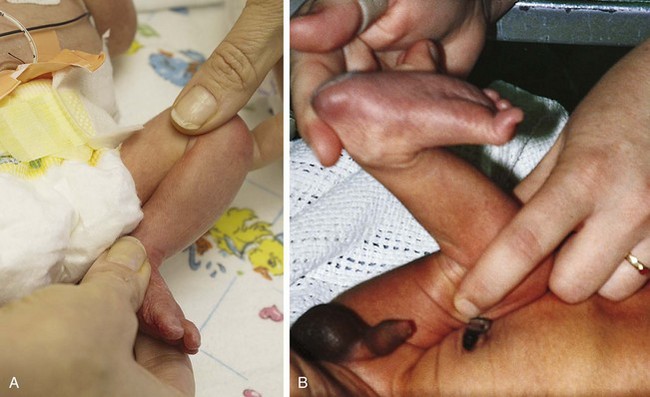
The rooting reflex may be elicited by lightly stimulating the infant’s cheek and observing the reflexive attempts to bring the stimulating object to the mouth. The sucking reflex is activated by placing an object in the infant’s mouth and observing the sucking movements. In the grasp reflex (Figs. 2-16 and 2-17), transverse stimulation of the midpalm (without touching the back of the hand) or midsole leads to flexion of the digits or toes around the examiner’s fingers.
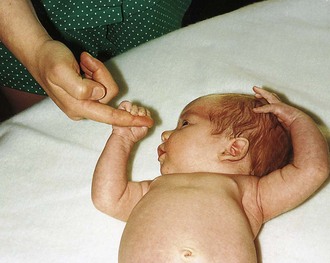
Figure 2-16 Grasp reflex (palm). Transverse stimulation of the midpalm leads to a grasp by the infant.
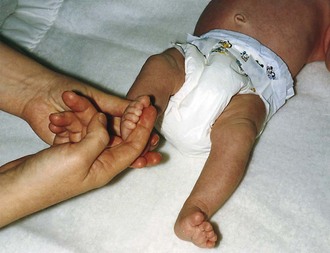
Figure 2-17 Grasp reflex (sole). Transverse stimulation of the midsole triggers a grasp by the infant.
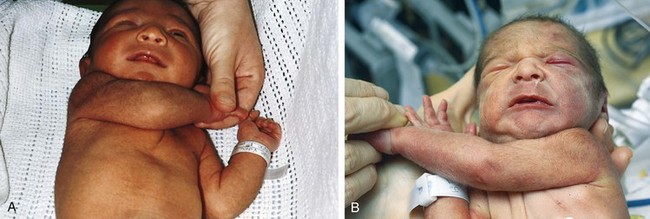
The Moro reflex (Fig. 2-18, A and B) evaluates vestibular maturation and the relationship between flexor and extensor tone. Elicitation of the reflex involves a short (10 cm), sudden drop of the head when the infant is supine. The full response involves extension of the arms, “fanning” of the fingers, and then upper extremity flexion followed by a cry. An incomplete but identifiable reflex becomes apparent at approximately 32 weeks’ gestation, and by 38 weeks it is essentially complete. Very immature infants demonstrate extension of the arms and fingers but do not show true flexion or make a sustained cry. Marked asymmetry of response may be associated with focal neurologic impairment.
Abnormalities of Growth
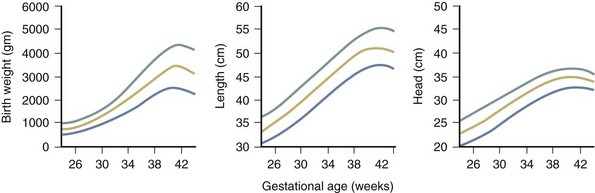
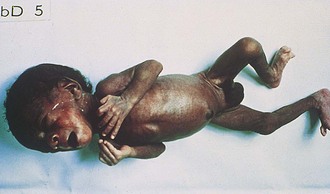
Intrauterine growth restriction (IUGR), a deviation in the expected fetal growth pattern, complicates up to 8% of all pregnancies, and is associated with an increase in perinatal morbidity and mortality. Infants with IUGR may appear long and thin and often have an obvious loss of subcutaneous tissue, which is best seen as redundant skin folds over the buttocks, thighs, and knees. The etiology of IUGR is multifactorial and includes fetal, placental, or maternal factors that inhibit normal fetal growth. The conditions for IUGR and SGA (small for gestational age) are related but not synonymous. The diagnosis of SGA is based on population norms and includes infants who weigh less than a predetermined cutoff value (Fig. 2-19). There is no universal agreement on the definition of the SGA or LGA (large for gestational age) infant. Similarly, appropriate for gestational age (AGA) infants have growth parameters within 2 standard deviations of the mean, or between the 10th and 90th percentiles, or between the 3rd and 97th percentiles.
The relationship among weight, length, and head circumference can be useful in understanding the etiology of the small size (see Fig. 2-19). By comparing length or head circumference percentiles with the weight percentile at any given gestational age, the clinician can detect growth retardation even if the actual weight still falls within 2 standard deviations of normal. Conditions that affect growth during the third trimester of pregnancy, such as preeclampsia, tend to interfere with the normal acquisition of fatty tissue while sparing brain growth (and thus head circumference) and linear growth. These newborns have an asymmetrical form of growth retardation (Fig. 2-20). Often postmature infants (>42 weeks) have some decrease in weight compared with length or head circumference. Problems beginning earlier than the third trimester tend to produce generalized growth retardation because head circumference, weight, and length are affected to equivalent degrees. Historically, infants with symmetrical IUGR have higher rates of chromosomal disorders, dysmorphic syndromes, and congenital infection and are associated with higher rates of prematurity and neonatal mortality. In very premature infants, global decreases in growth often complicate assessment of gestational age because the tools are rather limited in babies born at 24 to 28 weeks’ gestation. A thorough investigation should be undertaken in any unexplained instance of growth retardation.
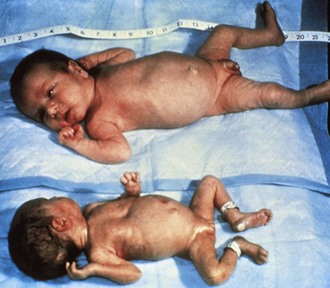
Multiple-gestation pregnancies often produce newborns that are premature and symmetrically small. Fetal growth decreases as the number of fetuses increases. Although multiple factors interfere with growth in these pregnancies, uterine constraint appears to occur when the combined fetal size approximates 3 kg. Size discordance (>10% difference in weight) between identical twins occurs because their placentas can share vascular connections, resulting in overperfusion of one twin and underperfusion with subsequent growth restriction of the other. Discordance may also occur in dizygotic twins (Fig. 2-21) if one has placental insufficiency. Rarely, only one twin will be afflicted with a chromosomal abnormality or congenital infection.
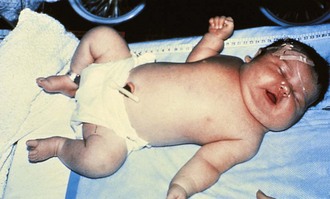
Newborns that are large for gestational age (LGA) are often the products of pregnancies in diabetic or “prediabetic” mothers. The effect is usually noted during the third trimester, with infants at term who weigh more than 4 kg (8 lb 13 oz). Weight is the most affected parameter, but length and head circumference are often increased as well. Infants of diabetic mothers are often identifiable by macrosomia, round facies (Fig. 2-22), and sometimes plethora and hirsutism (especially of the pinnae). Maternal hyperglycemia causes glycogen deposition in the newborn, resulting in visceromegaly, most notable in the liver and heart. Although babies weighing more than 8 pounds are more likely to be from diabetic pregnancies, a significant number of large full-term newborns are the product of normal pregnancies. Nevertheless, all LGA infants should be routinely screened for hypoglycemia and their mothers investigated for the possibility of undiagnosed diabetes mellitus.
Two fairly unusual syndromes can also cause excessive size: (1) cerebral gigantism, or Sotos syndrome, with macrosomia, macrocephaly, large hands and feet, poor coordination, and variable mental deficiency; and (2) Beckwith-Wiedemann syndrome with macrosomia, macroglossia, omphalocele, linear ear fissures, and neonatal hypoglycemia (see Chapter 9).
Placenta
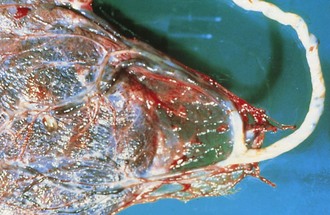
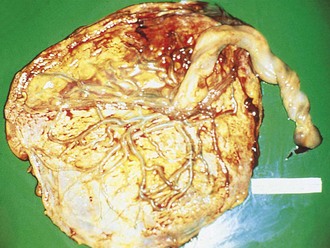
The insertion of the umbilical cord into the placenta, which can be central, eccentric, marginal, or velamentous, can be important in understanding unexplained asphyxia or blood loss. In a velamentous insertion (Fig. 2-23), the cord is inserted into the membranes rather than into the placental disk, leaving the umbilical vessels unprotected for a variable distance. These vessels are more prone to rupture, with resultant fetal hemorrhage (vasa previa).
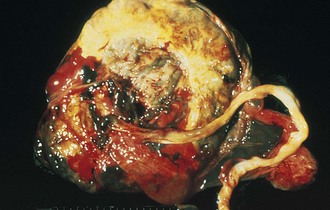
At times, placentation itself is abnormal. In a circumvallate placenta (Fig. 2-24), the villous tissue projects beyond the chorionic surface, with a hyalinized fold at the edge of the chorionic plate. This type of placentation may cause antepartum bleeding, premature labor, and increased perinatal mortality.
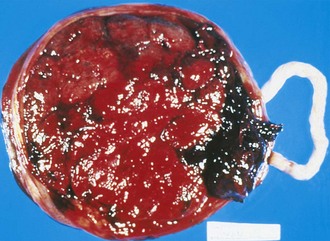
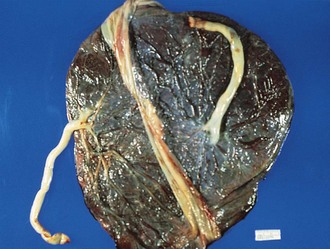
Premature placental separation (abruptio placentae) can lead to an accumulation of blood behind the placenta (Fig. 2-25). Although the bleeding is usually of maternal origin, rare fetal blood loss may also occur. Large abruptions may lead to poor growth, fetal asphyxia, or even death. Distinguishing a true abruption, in which an adherent clot compresses the maternal surface, from the nonadherent collection of blood that forms on normal placental separation is important.
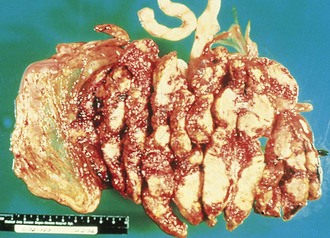
Placental infarctions (Fig. 2-26) tend to occur along the margin of the placenta, can vary in color from red to yellowish white, and are most common in pregnancies complicated by hypertension. Small placental infarcts (<30% of placental volume) are usually of little significance. However, large central infarcts can reduce the placental surface available for fetal oxygenation and nutrition and can result in aberrations in fetal growth.
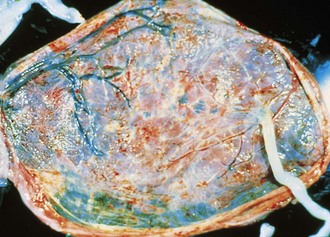
Chorioamnionitis (Fig. 2-27), or inflammation of the fetal membranes, is an immediate clue to potential neonatal infection. On gross examination the membranes lack their normal sheen and translucency, appearing gray or yellow. Inflammation, confirmable by microscopic examination, can also be found in the fetal vessels of the chorionic plate and umbilical cord.
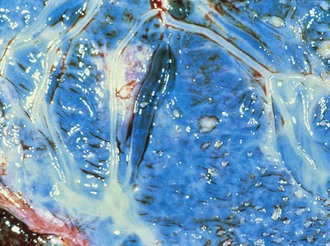
In pregnancies in which the quantity of amniotic fluid is decreased (oligohydramnios), examination of the amnion may also reveal shiny, gray, flat nodules known as amnion nodosum (Fig. 2-28). The presence of these nodules can be an immediate indication of renal dysfunction or renal agenesis in the newborn (or the newborn may have normal renal function). Because such infants may also have hypoplastic lungs and dysmorphic features (such as occurs in Potter syndrome), early diagnosis can be helpful to the physician and family.
In multiple-gestation deliveries, a careful placental evaluation is crucial to determine chorion number and to distinguish between monozygotic and dizygotic twins. The major distinction to be made is whether there is a single chorion, or outer layer of the fetal membranes. When twins with a single chorion are present in a single amniotic cavity (Fig. 2-29), monozygosity is ensured. For all practical purposes, a single chorion that bridges two amniotic sacs is also evidence of monozygotic twins. In this instance it is essential to examine the membranes at the site of connection of the two amniotic sacs. When two chorions and two amnions (or a total of four membranes at their interface) are present (Fig. 2-30), twins may be monozygotic or dizygotic. Approximately 36% of monozygotic twins are dichorionic. Monochorionic (MC) twin placentas, developed for a singleton pregnancy, may not adapt to the demands of twin circulations. The majority of MC twin placentas have connecting vessels, which account for the higher rates of complications.
Birth Trauma
Caput Succedaneum
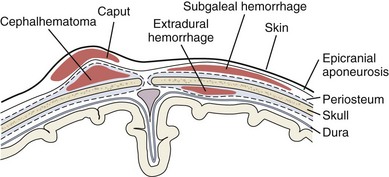
Normal transit of the fetal head through the birth canal induces molding of the skull and scalp edema, especially if labor is prolonged. The edema, which can be massive, is known as a caput succedaneum (Fig. 2-31). Much of this edema is present at birth and tends to overlie the occipital bones and portions of the parietal bones bilaterally. In some cases, bruising of the scalp may also be present (especially if a vacuum extractor was used). The presence of a caput requires no therapy, and spontaneous resolution within a few days is the rule. Distinguishing caput from a subgaleal (subaponeurotic) hematoma, a rare but serious complication of delivery, is important. A subgaleal hematoma is a collection of blood within scalp tissues extending beneath the epicranial aponeurosis. Palpation of a large caput succedaneum reveals firm, nonpitting swelling. In contrast, the cranial swelling of subgaleal bleeding is boggy due to the palpation of clotted blood just beneath the epicranial aponeurosis (Fig. 2-32). The collection of blood in this potential space can be quite large, and these infants must be monitored for signs of hypovolemia. Serial examinations, which can include measurement of head circumference and hematocrit, are important to identify ongoing blood loss.
Cephalhematoma
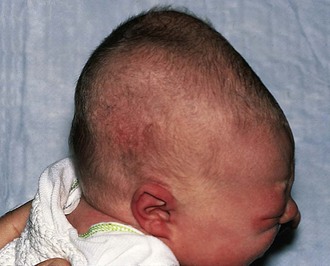
Often, confusion arises between the diagnosis of a caput and that of a cephalhematoma. The latter is a localized collection of blood beneath the periosteum of one of the calvarial bones; it may be bilateral, but is most often unilateral (Fig. 2-33). It is distinguished from a caput by the fact that its borders are limited by suture lines, usually those surrounding the parietal bones (see Fig. 2-32). However, diagnosis can be difficult in the immediate newborn period, when there may be overlying scalp edema. On palpation, the border may feel elevated and the center depressed. Most patients have an uncomplicated course of slow resolution over one or more months, although calcification may occur. On occasion, these infants may develop jaundice from the breakdown and resorption of the large hematoma. Underlying hairline skull fractures occur with some regularity but are rarely of clinical significance. The exception is the uncommon development of a leptomeningeal cyst. Radiologic investigation for an underlying depressed fracture is indicated in infants whose histories suggest significant trauma and those having depressed levels of consciousness or neurologic abnormalities on examination. Infection is another potentially serious but rare complication, which is more likely when the integrity of the overlying skin is broken.
Clavicle Fracture
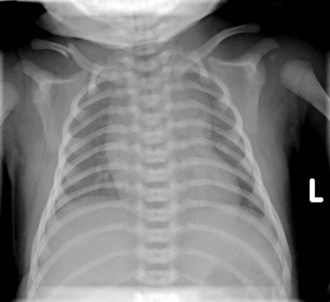
Fracture of a clavicle can occur during delivery when the infant is large, in breech position, or if there is fetal distress requiring rapid extraction. If undisplaced, the fracture may not be painful, and the infant may be asymptomatic (Fig. 2-34). The diagnosis may be suspected by palpation of crepitus or an asymmetrical Moro reflex. If there is pain or discomfort with routine handling, the fracture can be treated by immobilization of the ipsilateral limb and shoulder with the elbow flexed 90 degrees. Immobilization can be discontinued when a callus is palpable at 8 to 10 days. Many nondisplaced clavicle fractures are not diagnosed until the first newborn outpatient follow-up visit, when a large, firm callus may be palpated along the clavicle. If the child has an otherwise normal physical and neurologic examination at this time, a radiograph is not indicated. Radiographs would be indicated to help differentiate whether decreased arm movement is secondary to pain (clavicle fracture) or nerve injury (Erb’s palsy).
Meconium Staining
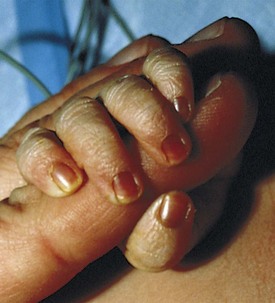
Meconium is noted in the amniotic fluid in as many as 10% of deliveries. The meconium may have been recently expelled or may have been present in the amniotic fluid for hours or days. Because the timing of the passage of meconium may have significance for the diagnosis of fetal distress, it is useful to examine infants for the presence of meconium staining. It takes at least 4 to 6 hours of contact before staining of the umbilicus, skin, and nails occurs (Fig. 2-35). Often, the meconium-stained infant is postmature and has diffuse peeling of the skin and a shriveled, stained umbilical cord.
Bruises and Petechiae
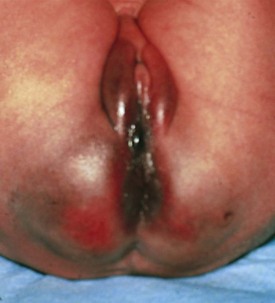
Superficial bruising can occur when delivery is difficult. This is relatively common with breech presentations (Fig. 2-36) and can include swelling and discoloration of the labia or scrotum (to be distinguished from an incarcerated inguinal hernia). When bruises are extensive, significant secondary jaundice may develop as the extravasated blood is broken down and resorbed. In an infant in whom a nuchal cord is found at delivery, the presence of diffuse petechiae around the head and neck is common and does not warrant further investigation. In addition, petechiae found on the presenting body part are normal. The appearance of new bruises or petechiae after delivery should alert the physician and nurse to the possibility of a bleeding disorder or infection.
Fat Necrosis
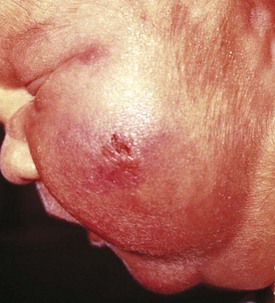
Many infants delivered with the aid of forceps show forceps marks after delivery. These marks tend to fade over 24 to 48 hours. On occasion, a well-circumscribed, firm nodule with purplish discoloration may appear at the site of a forceps mark. This may represent fat necrosis (Fig. 2-37) and resolves spontaneously over weeks to months. The phenomenon may also occur at other sites of trauma. Affected infants may develop symptomatic hypercalcemia.
Nasal Deformities
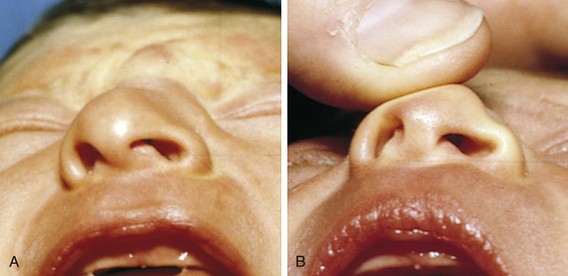
Abnormalities of the nose are common after delivery, the majority consisting of transient flattening or twisting induced during transit through the birth canal. Less than 1% of nasal deformities are due to actual dislocations of the triangular cartilage of the nasal septum. These can be differentiated from positional deformities by manually moving the septum to the midline and observing the resultant shape of the nares. In a true dislocation, marked asymmetry of the nares persists (Fig. 2-38). Returning the septum to its proper position can be accomplished in the nursery with the guidance of an otolaryngologist. Failure to recognize and treat dislocation may lead to permanent deformity.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree