Hypothermia9
Perinatal asphyxia8
Prolonged use of antibiotics from birth despite negative cultures12-14
Blood transfusion15,16
Formula feeding8
A meta-analysis of 11 retrospective case-control studies and one cohort study found that recent blood transfusion was associated with NEC and that neonates with transfusion-associated NEC compared with neonates with NEC but no recent transfusion were younger, weighed less and were more likely to have PDA and to be receiving ventilator support.15 A Canadian case-control study found that 15.5% of infants with NEC had been transfused in the previous 2 days compared with 7.7% of age-matched controls.16 This suggests blood transfusion should be avoided if possible in ventilated infants with PDA.
Full-term infants almost always develop their NEC in the first week of life and while some have risk factors such as congenital heart disease around half have no identifiable risk factor.17,18
11.1.1.1 Feeding and necrotizing enterocolitis
Breast milk protects against NEC. In a prospective multicenter study, NEC developed in 5.5% of 926 pre-term infants and was 6–10 times more common in infants fed exclusively with formula than exclusively with breast milk and 3 times more common in those who received mixed formula and breast milk.19 The increased risk of NEC with mixed feeding compared to breast milk alone suggests there is something harmful in formula feeds as well as protective in breast milk. In this study pasteurized donor breast milk was as protective as raw mother’s milk.19
In a Cochrane systematic review and meta-analysis of data from five trials comparing donor breast milk with pre-term formula in pre-term infants, NEC was significantly increased in the formula-fed group (typical RR 2.5, 95% CI 1.2, 5.1; number needed to harm = 33, 95% CI 17, 100).20
In some epidemiologic case-control studies, infants with NEC were more likely to have reached full feeds than control infants,10,13 although other studies disagree.5 However, increasing scientific evidence suggests delaying enteral feeds or increasing their volume slowly does not prevent NEC. A Cochrane systematic review and meta-analysis of five randomized controlled trials (RCTs) in 600 infants <;1500 g found delayed introduction of enteral feeds to later than 5–7 days compared with ≤4 days after birth did not reduce NEC (typical RR 0.89, 95% CI 0.58–1.37) or all-cause mortality, but did delay the time to establish full enteral feeds.21
An RCT comparing minimal enteric feeds with advancing feeds in 141 pre-term infants was stopped early when 7 infants in the advancing group (10%) developed NEC compared with only one fed minimal volumes.22 However, subsequent studies have not confirmed this association. A Cochrane systematic review and meta-analysis of four trials (496 infants) found that slow advancement of enteral feeds compared with faster rates of increase did not prevent NEC (typical RR 0.91, 95% CI 0.47–1.75) or reduce all-cause mortality but infants on slow advancement took longer to regain birth weight and to establish full enteral feeding.23
11.1.2 Pathogenesis of necrotizing enterocolitis
NEC is characterized histopathologically by extensive inflammation of the gut with ischaemia and necrosis of the intestinal epithelium. Even if ischaemia contributes to the necrosis, it does not explain the inflammation. The length and depth of the necrosis vary. The classical sites of NEC are the terminal ileum and caecum, which are the most common sites if perforation occurs,24 but the whole bowel can be involved. The molecular and cellular mechanisms causing the pathologic changes are poorly understood. Various exogenous and endogenous mediators such as endotoxin (lipopolysaccharide), inflammatory cytokines, platelet activating factor and nitric oxide have been postulated to have a role in pathogenesis.25 There is intense interest in molecular signalling in NEC: nitric oxide may play a significant role as a molecular signalling ‘hub’ in the failure of the gut barrier in NEC,25 while activation of cyclooxygenase-2, a critical enzyme in the biosynthesis of prostanoids, may be a critical step in pathogenesis.26
11.1.3 Clinical presentation of necrotizing enterocolitis
Pre-term babies with NEC usually present between the second and eighth week after birth: in one study the median age of presentation was 13 days.2,5 In contrast, full-term babies almost always present in the first week, with a mean age of presentation around 4–5 days of age.5,17,18
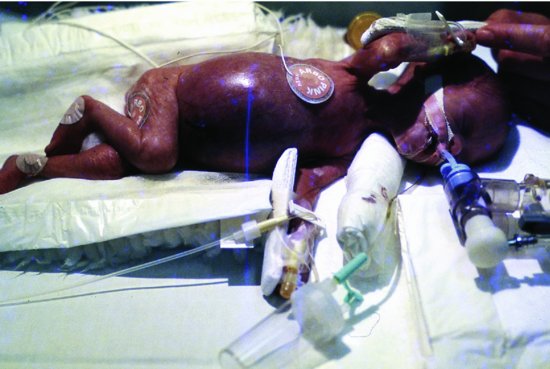
The clinical presentation may be insidious or fulminant. The insidious presentation is with intolerance of enteral feeds, abdominal distension, tenderness to palpation and possibly with vomiting, apnoea, bradycardia, irritability or lethargy and temperature instability. The fulminant presentation is with gross abdominal distension and shock, with or without bloody diarrhoea. The abdomen may be tense, red and shiny as well as distended (Figure 11.1) Perforation causes signs of peritonism with a rigid abdomen as well as shock. In severe cases there may be oedema and induration of the abdominal wall. Abdominal crepitus is a rare, sinister sign.
A common clinical problem is assessing whether or not a pre-term infant who becomes intolerant of feeds with increasing gastric aspirates has NEC. If the abdomen is soft and non-tender, NEC is unlikely, whereas a tender distended abdomen is highly suspicious. In either case, the radiologic appearance may help, whereas blood tests are generally unhelpful.
11.1.4 Radiology of necrotizing enterocolitis
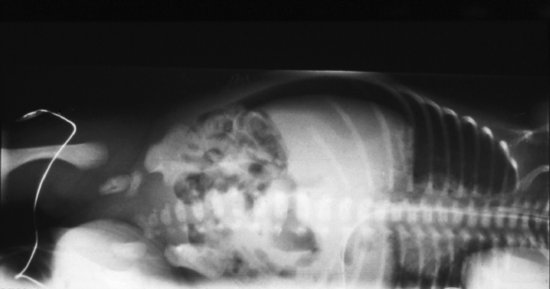
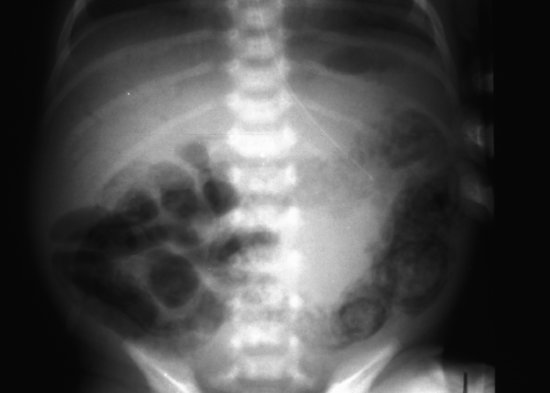
The early radiographic signs of NEC are non-specific, showing abdominal distension and generalized bowel dilatation, suggestive but not diagnostic of ileus. Later more overt radiographic signs of NEC may include bowel wall oedema, a fixed loop on repeated radiographs, an increasingly gasless abdomen, intramural gas (Figures 11.2 and 11.3),air in the biliary tree (Figure 11.4) and free air due to perforation (Figure 11.5) As with many radiographic investigations, there is relatively poor agreement between experts on the interpretation of radiographs27,28 and indeed, a radiologist who read the same radiographs twice 3–6 months apart, often interpreted them differently.27 Reassuringly, trained experts fared better than in-training observers.27
Figure 11.2 Necrotizing enterocolitis: extensive intramural gas throughout transverse colon and bowel wall oedema.
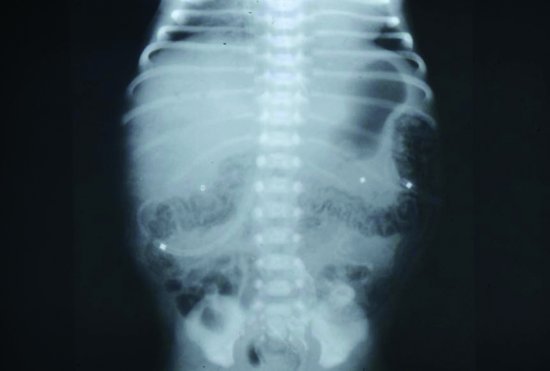
Figure 11.3 Close-up of left side of abdomen showing double shadow of intramural gas in descending colon.
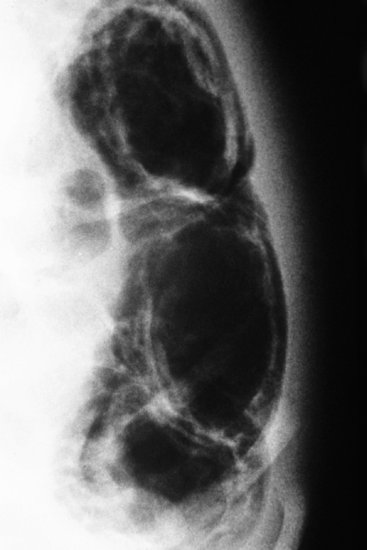
Figure 11.4 Necrotizing enterocolitis: intramural gas in descending colon and air in liver outlining biliary tree (portal venous gas).
Even more reassuringly, a Duke University study using a 10-point scoring scale to score radiographs found much better inter- and intra-observer agreement.29 In a subsequent study, local experts used the scale to assign radiographic scores in a blinded fashion to radiographs from infants with suspected NEC, based on the presence or absence of fixed bowel loops, pneumatosis intestinalis (intramural gas) or portal venous gas.30 Patients with higher scores were more likely to need surgery and the scale performed well when assessed by receiver-operating characteristic curve.
In a South Korean study, neonates with NEC and bowel distension without pneumatosis on plain radiograph had either echogenic dots or dense granular echogenicities in the bowel wall.31 In a Canadian study, infants treated medically were less likely to have abnormal ultrasound appearance than those treated surgically. An adverse outcome was associated with ultrasound findings of free gas, focal fluid collections or three or more of increased bowel wall echogenicity, absent bowel perfusion, portal venous gas, bowel wall thinning, bowel wall thickening, free fluid with echoes and intramural gas.32
Although NEC is the single most common cause of pneumoperitoneum, even in developing countries,33 pneumoperitoneum can also occur due to isolated perforation of the bowel not caused by NEC.
11.1.5 Laboratory tests in necrotizing enterocolitis
Blood culture should always be performed in suspected NEC: up to a third of infants with NEC are bacteraemic. Meningitis occurs in about 1% of cases of NEC. LP should be considered prospectively and also considered retrospectively if blood cultures grow an organism likely to cause meningitis.
A full blood count will identify thrombocytopaenia and/or neutropoenia. Acute phase reactants, generally raised in NEC, do not distinguish NEC from other causes of inflammation or sepsis.
Stool microscopy may reveal multiple Gram-positive cocci in staphylococcal enterocolitis which can mimic NEC. Stool bacterial cultures in suspected NEC are of limited value except during a possible outbreak. Clostridium difficile toxin may be found in asymptomatic infants and should not be routinely sought. However, in an apparent outbreak of NEC, C. difficile toxin testing is advisable as are stool viral studies because of the infection control implications.
11.1.6 Treatment of necrotizing enterocolitis
The empiric antibiotic treatment of NEC is based on the need to cover bowel organisms. The most common organisms isolated from blood culture in case series of NEC are Gram-negative bacilli (E. coli, Klebsiella, Pseudomonas and Enterobacter),8,34-36 but other important pathogens include S. aureus,4 CoNS3 and enterococci, while anaerobes are occasionally isolated.8,34-36
There are no RCTs of antibiotic treatment of NEC. A sequential study from Canada compared ampicillin and gentamicin with cefotaxime and vancomycin.37 The incidence of culture-positive peritonitis and mortality in infants <;2200 g was lower in the second study period, but the sequential nature of the study casts doubt on whether this was due to the different antibiotic regimens.37
The empiric antibiotic regimen for NEC is generally recommended to include anti-staphylococcal cover (vancomycin if MRSA is prevalent or the infant is colonized) and appropriate Gram-negative cover. Clindamycin and gentamicin are often used, clindamycin providing cover against Gram-positive and most anaerobic organisms and gentamicin against Gram-negative bacilli. Vancomycin and gentamicin are preferred if there is a significant risk of MRSA and many would add metronidazole to provide anaerobic cover. Local epidemiology may also inform the choice of antibiotics.
If meningitis is clinically suspected but LP not performed, empiric antibiotic cover should obviously include antibiotics that penetrate CSF.
If blood cultures are positive, the antibiotic regimen should be modified accordingly and the need for LP reconsidered carefully.
The optimal duration of antibiotic therapy for NEC has not been studied formally. It is usual to continue antibiotics until the infant is tolerating feeds again. Bacteraemia is treated for 7–10 days (see Chapter 5) and meningitis for 14–21 days (see Chapters 5 and 7).
Ancillary management includes careful attention to the need for circulatory and cardiorespiratory support, blood and platelet transfusions and monitoring and correcting abnormalities of fluid and electrolyte balance and blood sugar. There is no evidence for or against adjunctive treatment of NEC with lactoferrin38 or any other immunomodulatory therapy.
A critical consideration is the need for surgical intervention; an early surgical opinion and close follow-up are important. The surgical alternatives are open laparotomy and peritoneal drainage. A Cochrane systematic review39 found only two trials (185 patients) comparing peritoneal drainage and laparotomy. These found no difference in mortality or need for parenteral nutrition, although in one study time to full enteral feeds was prolonged in the peritoneal drainage group.40,41
11.1.7 Outcome of necrotizing enterocolitis
Mortality depends on severity, but is around 20–30% of severe cases, generally higher the lower the gestation, and higher for infants managed surgically than medically.19,41-43 Interestingly, however, in a study of infants <;1500 g who required surgery, the mortality was 25% in infants <;1000 g and those 1000–1500 g. The only variables associated with increased mortality were pan-necrosis and the length of the segment of necrotic bowel.44
In a follow-up study of infants <;1000 g, surgically but not medically managed infants with NEC had a significantly increased risk of periventricular leukomalacia, IQ <;70, neurodevelopmental impairment and growth retardation than those without NEC.45
In a systematic review of 10 studies of infants 45% of infants with NEC were neurodevelopmentally impaired and they were significantly more likely than gestational age-matched controls to have impaired neurodevelopment (OR 1.6, 95% CI 1.3–2.0), cerebral palsy (OR 1.5, 95% CI 1.2–2.0), visual (OR 2.3, 95% CI 1.0–5.1), cognitive (OR 1.7, 95% CI 1.4–2.2) and psychomotor impairment (OR 1.7, 95% CI 1.3–2.2). The risk of disability increased with severity of NEC.46
Infants in the UK ORACLE study who developed NEC had an increased risk of all grades of impairment at age 7 years.47 Children with NEC were four times more likely to suffer bowel problems (short bowel syndrome, strictures, fistulas and anastomotic leak, presence of stoma) than non-NEC children and had significant long-term motor, sensory and cognitive impairment.47
11.1.8 Prevention of necrotizing enterocolitis
Table 11.2 Strategies to prevent necrotizing enterocolitis.
| Strong evidence from meta-analyses of randomized controlled trials (RCTs) Use breast milk (mother or donor) and avoid formula feeds20 Prophylactic oral probiotics9 (Section 11.1.8) |
Convincing evidence from large observational studies
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|