(1)
Bordeaux, France
For in vitro fertilization (IVF), the process of ovarian stimulation includes some unique yet essential contrasts to the methods for classic mono- or pauci-follicular stimulations.
First and foremost, this process is a multifollicular stimulation. Even if only a single embryo is to be transferred, IVF typically seeks to collect several oocytes in order to provide an optimal choice of embryo for transfer, as well as to enable frozen preservation of supernumerary high quality embryos for possible transfer at a later time.
Therefore, this procedure should be considered as a deliberate ovarian hyperstimulation. It should be fully expected, if hCG is administered, that the excessive quantity of follicles of all sizes, developing in both ovaries, together with the resulting estrogen secretion, will expose the patient to well-identified risks for an ovarian hyperstimulation syndrome. A safe process is made possible solely because oocyte harvesting evacuates the follicular contents and diminishes the chain of events leading to an OHSS. This procedure is in effect a controlled expansion of the safety limits.
Ovarian stimulation is only one of several aspects necessary for a successful outcome from IVF. Chances for pregnancy are very dependent on high standards and quality in the embryology laboratory. While a high-grade laboratory can compensate to an extent for suboptimal oocyte quality, the reverse is not true. The best-run stimulation protocol will be of little value in the face of inadequate embyrology procedures. For this reason, stimulation quality in IVF correlates less well with successful pregnancy rates than do most classic stimulations.
This multifollicular stimulation, or rather, this controlled ovarian hyperstimulation (COH) is realized by manipulating two of the usual rules that control follicular recruitment and development (Fig. 12.1): (1) Administration of supra-physiologic gonadotropin doses as soon as the first days of the cycle, which quickly exceeds the FSH threshold and recruits more elements of the follicular pool. (2) Continuation of these high doses throughout the stimulation period to prevent closure of the FSH window, thereby sustaining a complete development and maturation of most of the recruited follicles.


Fig. 12.1
Comparison of the classical and multifollicular ovarian stimulation
12.1 Evolution of Ovarian Stimulation Protocols for IVF
Having abandoned the use of clomiphene for ovarian stimulation, Edwards and Steptoe turned next to a low dose gonadotropin stimulation, but left this approach as well because they felt that it led to implantation failures. Their first successful IVF pregnancy in 1978 was actually the result of a spontaneous ovulation that simply involved monitoring the urinary peak of LH to identify the proper moment for oocyte harvest [1]. Once the feasibility of IVF was demonstrated, additional teams decided to stimulate ovulation with the goal of producing more oocytes and more suitable embryos for transfer.
12.1.1 Stimulation Protocols
Initially, two distinct stimulation protocols came into use [2]:
The Clomiphene–HMG protocol, more commonly used in Europe: Oral clomiphene citrate, 100 mg daily, was administered for 5 days, starting on CD2, followed by injections of 225 IU HMG on CD 3, 5, and 7, and with the initial first monitoring assessment conducted on CD 9.
The HMG–Only protocol, more commonly adopted in the USA: This model was notably developed and used by Drs. Georgiana and Howard Jones, and consisted simply of injections with 150–225 IU HMG daily from CD 2 or 3, and initial monitoring controls made on CD 7–8.
In fact, both protocols had a pair of blatant drawbacks: one was that it resulted in the production of asynchronous follicles of many sizes. In addition, untimely LH surges promoted by supra-physiologic estrogen secretion from the numerous follicles led to premature luteinization and/or to an ovulatory process fraught with difficulties in choosing the precise timing for oocyte harvest. As a result many cycles had to be cancelled.
Furthermore, management of these protocols was somewhat challenging. hCG administration should ideally be done at the precise moment when sufficient numbers of follicles are mature, but before a spontaneous gonadotropin surge provoked by the most advanced follicle begins to halt the development of intermediary follicles. This difficult task often led to a disappointing oocyte yield or to an overly high rate, some 15 %, of cancelled cycles. Successful pregnancy rates, taking into account all degrees of hCG elevation, were only 15–25 % per transfer, although poor performance of embryology laboratories during this era undoubtedly played a role in overall results. At the present time one should expect rates at least twice as high, now that these protocols are enhanced by GnRH antagonists.
12.1.2 Use of GnRH Agonists
The appearance of GnRH agonists in 1986 revolutionized protocols of ovarian stimulation for IVF purposes because the agonists suppress the potential for LH surges and thereby improve management of the stimulation process. Out of this principle, two new protocol designs appeared at virtually the same moment:
The Short Agonist Protocol, that exploited the initial “flare-up” effect of a short-lived agonist. It quickly fell from favor because its pregnancy rates were only marginally better than those of previous protocols not using GnRH analogs. While the long agonist protocol rates are now clearly superior, the short agonist approach remains as a possible last resort for certain poor responders.
The Long Agonist Protocol, that can be considered as an ovarian stimulation after a preliminary desensitization. This approach has resulted in a clear augmentation of successful pregnancy rates, and has now risen to become the gold standard of ovarian stimulation for IVF purposes.
In contrast to earlier protocols, a more homogeneous follicular cohort results from this long agonist protocol. To be sure, a distinctly greater total gonadotropin dose is necessary to stimulate development and maturation of follicles in a previously desensitized ovary. However, the absence of premature LH surges together with the homogeneous follicle cohort allows selection of more oocytes and leads to higher pregnancy rates. The period for follicle recruitment is also extended. It must also be acknowledged that follicular cohort sizes and plasma estradiol levels are much higher than previously known during stimulation protocols, and this is accompanied by a higher risk for ovarian hyperstimulation.
Because of these enhanced risks for hyperstimulation and also multiple pregnancies following the transfer of several embryos, and also because of modern success with embryo freezing, clinicians are now being urged to consider a single embryo transfer (SET) and to freeze the supernumerary good quality embryos. This reduces the need for multifollicular stimulation cohorts and results in milder (“soft,” “friendly”) stimulation protocols.
The appearance of GnRH antagonists in 1999 could not have come at a better time to enhance this strategy because daily antagonist administration only prevents premature LH surges. Without the preliminary desensitization, the follicular cohort becomes more heterogeneous and smaller in number than with GnRH agonists. Despite a smaller number of oocytes, pregnancy rates remain comparable to those from agonist use but are made possible within a more moderate stimulation protocol. Moreover, risks for ovarian hyperstimulation are dramatically reduced because ovulation may be triggered when necessary using a short lived GnRH agonist.
Use of GnRH antagonists finally brings in the advantages of earlier multifollicular stimulation protocols that were in use before the appearance of GnRH agonists. Thanks to adequate control of the LH levels, it is once again possible to develop a sufficient number of mature oocytes and realize customary pregnancy success rates, but without the drawbacks of the long agonist protocol. On the other hand, management of antagonist stimulation is somewhat more challenging than with agonist protocols. Deciding on the precise moment to introduce the antagonist, at least in the flexible protocol, becomes crucial.
12.2 The Multifollicular Stimulation Protocol for IVF
At the present time, follicular stimulation for IVF purposes (controlled ovarian hyperstimulation, COH) should use both gonadotropins and a GnRH analogue in tandem to prevent a misplaced ovulation or a premature luteinization.
12.2.1 Preliminary Steps
Evaluations and preparations for stimulation are not different from those for a classic stimulation; numerous factors such as tobacco use and body weight should be taken into consideration and corrected whenever possible. Similarly, the clinician must be aware of any chronic medical conditions that could be affected by this type of procedure or by pregnancy itself. One should also investigate for a patient or family history of clotting disorders.
Evaluation of the patient’s ovarian reserve is mandatory for COH, because this assessment helps to anticipate excessive and/or untoward ovarian responses, and it provides guidance for selection of the starting gonadotropin dose. The two cornerstone indices of ovarian reserve are the AFC and plasma AMH. These measures usually vary in the same direction, but there is not yet a true consensus of normal ranges for these parameters. That said, an excessive response to stimulation should be anticipated when the total AFC is more than 24 follicles and/or the AMH level is above 6 ng/ml. Conversely, an insufficient response is likely when the total AFC is less than six follicles and/or the AMH level is lower than 1.0 ng/ml. It should be noted that while the AMH level correlates to some extent with the size of the recruited follicular cohort, it cannot predict oocyte quality or the chances for a successful pregnancy [3].
Several algorithms with multifactorial calculations have been proposed to establish the proper starting dose for each patient, but these typically approach only a probabilistic level [4]. Nevertheless the initial dose, which will be maintained for the first 5–6 stimulation days, is highly important for a harvest of 6–15 mature oocytes, the optimal number for achieving a live birth [5]. An insufficient starting dose will recruit a low follicle number that will not be corrected by further increases of posology; an excessive dose will provoke growth of too many follicles and a decrease of subsequent dosing may become very difficult to manage.
12.2.2 GnRH Analogues
Gonadotropin releasing hormone (GnRH, gonadorelin) is a decapeptide secreted in a pulsatile manner by hypothalamic neurons located primarily in the preoptic nucleus that project to the median eminence for secretion into portal vessels. Arcuate neurons project dopaminergic fibers that modulate GnRH release and suppress prolactin release. Upon transport via hypothalamic-pituitary portal vessels, GnRH binds to specific receptors on pituitary gonadotrophs. A pulsatile neuropeptide secretion signals a similar pattern of FSH and LH release that controls plasma gonadotropin levels, their circulating ratio, and also their relative bioactivity according to variations of their isoform composition.
The pituitary gonadotroph GnRH receptor is a G-coupled protein whose function follows the basic principle of desensitization. In order to exert a stimulating effect upon the gonadotropin secretion, the occupation of these receptors must be episodic, as is precisely the case with endogenous pulsatile GnRH secretion. When receptor occupation is maintained by continuous administration of GnRH or a high-affinity analogue, a tachyphylaxis occurs that terminates additional gonadotropin release. The intracellular subunits of the G-coupled GnRH receptor must reassemble in order to respond to another stimulus, but cannot do so while the peptide ligand remains bound to the outside binding domain. In order to be effective, GnRH administration must be pulsatile and this is accomplished through the use of a programmed pump. Continuous administration of GnRH is also possible if one wishes to terminate or down-regulate further gonadotropin release in order to reduce gonadal activity (Fig. 12.2).


Fig. 12.2
The opposite effects of gonadorelin in accordance with its mode of administration
Substitution of certain amino-acids of the GnRH decapeptide sequence creates analogues having different biological properties than the native hormone. However, the three first amino acids of the sequence are necessary for biological activity and the eight residue is essential for high affinity receptor binding (Fig. 12.3).


Fig. 12.3
Gonadorelin structure
12.2.3 GnRH Agonists
A simple substitution of the l-alanine residue at position 6 or the l-glycine at position 10 with the respective dextrorotary enantiomer creates a potent agonist molecule because it gains an enhanced affinity for the GnRH receptor and the structure resists degradation by plasma peptidases. These modified peptides retain a longer bioactive half-life and are effective at lower doses, which explains the contradictory effects of long acting agonists on gonadotropin secretion. An initial potent stimulation of gonadotropin release (the “flare up” effect) is quickly followed by a secondary inhibition due to residual occupation of receptors by the agonist. Continuous administration of native GnRH also produces a refractory desensitization of gonadotrophs. Similarly, prolonged administration of these modified agonists, either daily (in nasal sprays or by subcutaneous administration) or as injected depot preparations, results in FSH-LH suppression and a concomitant anovulation and reduction of ovarian steroids. This property is being used to advantage in various estrogen-dependent clinical conditions, from precocious puberty to endometriosis to hormone-dependent malignancy (Table 12.1).
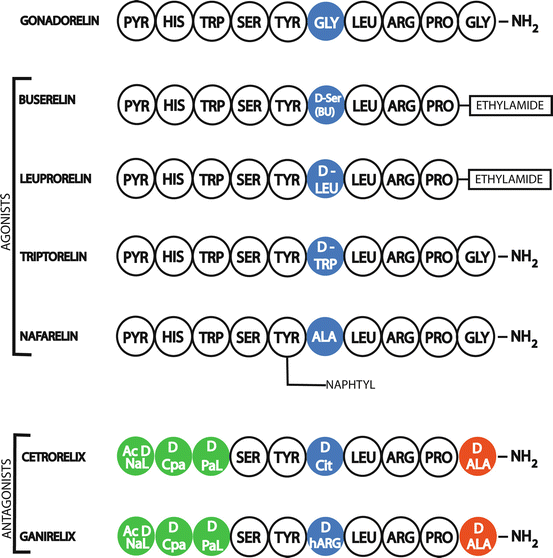
Table 12.1
Structures of gonadotropin releasing hormone (GnRH, gonadorelin) and several analogs

12.2.4 GnRH Antagonists
Substitution of the amino acids at positions 1, 2 and 3 of the decapeptide sequence results in loss of most of the biological activity, but the property of high-affinity receptor binding is preserved. This type of analogue acts as a GnRH antagonist: it binds to the receptor without an initial flare up effect while it prevents native GnRH from gaining receptor access (Fig. 12.4). A single administration has only a brief duration of action (about 24 h) and is reversed by use of GnRH itself or with one of the synthetic short lived agonists.


Fig. 12.4
Modes of action of GnRH agonists and antagonists
12.2.5 COH with GnRH Agonists
Use of GnRH agonists was introduced into multifollicular stimulation protocols for IVF purposes with the aim of preventing inappropriate LH surges. This controls the number of follicles reaching full maturity without risk that the more advanced ones would trigger a spontaneous ovulation. At present there are two protocols that differ in the timing of GnRH use:
The Short Protocol (Fig. 12.5). This utilizes only short-lived agonists, administered daily. Treatment starts on CD2 with the agonist alone for two days, then gonadotropin injections are added from CD3 until ovulation. The two important effects of the agonist are at work:


Fig. 12.5
Design of the short agonist protocol
The initial stimulatory action provokes an FSH – LH surge that initiates follicular recruitment, roughly equivalent to a daily administration of 150 IU FSH.
The subsequent pituitary tachyphylaxis permits exogenous ovarian stimulation through a full maturity of the follicular cohort but removes risk for a premature endogenous LH surge.
The first monitoring control should be done on CD6, the 5th stimulation day of four days GnRH agonist with two days gonadotropin administration added. Thereafter, assessments should be conducted at 2–3 day intervals according to the ovarian response (Table 12.2).

Table 12.2
Management of short agonist multifollicular protocol with a stimulation target of 6–14 follicles after 2 days of FSH (see StimXpert)

The Long Protocol (Fig. 12.6). This design introduces a new concept in ovarian stimulation, by exploiting even more the principle of pituitary desensitization. Stimulation itself is begun only after the ovaries have been put to rest by the prolonged withdrawal of endogenous gonadotropins following the agonist-induced shutdown of pituitary FSH and LH. The initial GnRH-induced flare up effect is not exploited in this protocol (Table 12.3). Ovarian activity ceases following daily administration of a short-lived GnRH agonist, or after a single injection of a long-acting GnRH agonist that lasts upwards of 1 month. In the latter case, gonadotropin administration should begin during the second half of the agonist effect, so that a possible embryo is not exposed to the GnRH analogue. Accordingly, gonadotropin administration begins 15–20 days after the agonist is administered, when ovarian shutdown has been established, and the injections continue under agonist control until the ovarian triggering.



Fig. 12.6
Design of the long agonist protocol
Table 12.3
Management of long agonist multifollicular protocol with a stimulation target of 6–14 follicles after 5 days of FSH (see StimXpert)

Long-acting agonists may be administered on the first day of the cycle (a “follicular long-agonist” protocol), with the desensitization control and gonadotropin administration beginning on CD15. Alternatively, it may be started following ovulation (CD20) in the previous cycle (a “luteal long-agonist” protocol); in that case, desensitization control and gonadotropin administration should begin on CD 10 of the following cycle.
Both protocols produce comparable results. Observations of functional ovarian cysts on the day of desensitization control are undoubtedly less frequent after the luteal administration protocol. On the other hand, the follicular administration protocol is the approach of choice in cases of irregular cycles, because administration on CD 20 of the previous cycle might intervene just before a late spontaneous ovulation and thus disorganize the following menses.
When complete ovarian rest is assured and gonadotropin administration is started, it is possible to reduce the daily dose of a short-lived agonist by half without risking a pituitary “escape” before triggering ovulation. Although this method was initially proposed for cases of poor ovarian response, it may be extended to all the patients.
12.2.6 Choosing a GnRH Agonist
Long-acting depot agonists produce a more “depressed” pituitary gland than the ovarian rest obtained with daily injections of short-acting agonists. The latter usually leaves a basal level of gonadotropin secretion that daily agonist injections maintain at a minimal but not absent level. For this reason a greater total quantity of gonadotropins will be necessary to complete a stimulation cycle when long-acting formulations are used, because there is not even a low-level endogenous pituitary contribution.
None of the available GnRH agonists has been established as superior to any other insofar as pregnancy rates are concerned, and there are no reports of systematic prospective studies comparing one to another. On the other hand, patient responses may vary from one agonist to another, and a particular agonist may produce different results in different patients. This difference can be appreciated by examining the gonadotropin surge profile of the flare up effect, or by the comparative ability of various agonists to suppress FSH and LH in different patients. Thus one may consider changing to a different agonist in the same patient, from one COH to another.
Regardless of the ovarian appearance at ultrasound, pituitary suppression may be considered complete when plasma estradiol falls below 30 pg/ml. Ovarian ultrasound should reveal a number of follicles <6–8 mm accompanied by a thin linear uterine endometrium. Complete pituitary shutdown may not be reached by the time of the first examination. In these cases, continuation of the short agonist, or waiting several days longer after a depot long-acting agonist injection, may bring a more satisfactory ovarian rest.
It remains however possible that estradiol values will level off, or even increase while awaiting the moment to commence gonadotropin injections. In these cases, one may consider prolonging the cycle by immediate administration of another agonist and then repeating the assessment 15 days later. On rare occasions a paradoxical stimulatory effect of a depot agonist may occur, and a true ovarian hyperstimulation still remains possible. If ultrasound reveals numerous mature or pre-mature follicles in the presence of elevated serum estradiol levels, moving ahead with triggering the ovulation and retrieving oocytes seldom results in a successful pregnancy, and usually leads to a diminished chance for pregnancy in the patient [6]. In this situation it is preferable to cancel the cycle and prescribe 20 days of a potent oral progestin, whose anti-gonadotropic effect will return the ovaries to a resting state.
Once the desensitization is accomplished, ovarian stimulation may start, with an initial monitoring control made after 5–6 days of gonadotropin administration. Additional controls, basically intended to decide possible modifications of posology, are scheduled every two to three subsequent days. Two or three evaluations following the desensitization control will usually be necessary and sufficient.
12.2.7 Choosing a Gonadotropin Preparation
All of the available commercial preparations with an FSH effect produce essentially comparable pregnancy rates. The only question for clinicians is whether to add LH during the protocols, because use of GnRH agonists will suppress pituitary release of both gonadotropins. In fact, a large majority of patients maintain a residual, sufficient LH secretion, so administration of FSH alone should provide adequate ovarian stimulation. An essential minimal level for LH is estimated to be 1.2 IU/l. In theory, this identifies a need for serum LH monitoring, if a borderline endogenous LH secretion becomes insufficient for optimal follicular maturity. However, most LH assay kits are inadequately sensitive for accurate measures below 2 IU/l. Insufficient LH secretion may also be suspected when the follicular growth rate observed at ultrasound is rising faster than estradiol levels, but this kind of discrepancy may be very difficult to identify with a multifollicular cohort. The problem is manageable by changing the gonadotropin preparation and turning to an FSH + LH effect for successive cycles.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


