Chapter 6 Maternal Physiologic and Immunologic Adaptation to Pregnancy
 Normal Values in Pregnancy
Normal Values in Pregnancy
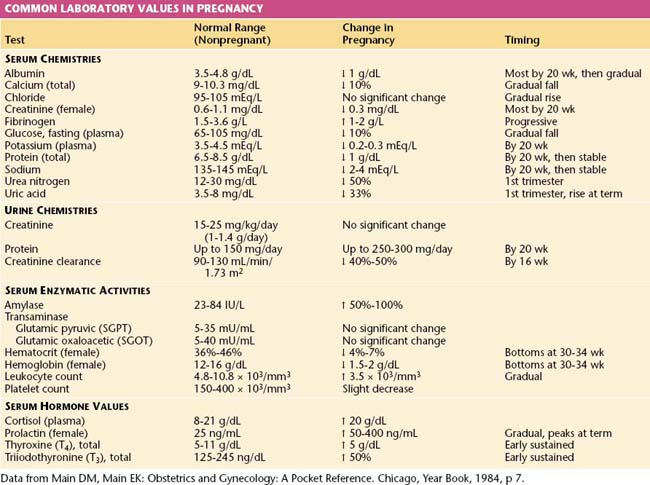
The normal values for several hematologic, biochemical, and physiologic indices during pregnancy differ markedly from those in the nonpregnant range and may also vary according to the duration of pregnancy. These alterations are shown in Table 6-1.
 Cardiovascular System
Cardiovascular System
CARDIAC OUTPUT
The hemodynamic changes associated with pregnancy are summarized in Table 6-2. Retention of sodium and water during pregnancy accounts for a total body water increase of 6 to 8 L, two thirds of which is located in the extravascular space. The total sodium accumulation averages 500 to 900 mEq by the time of delivery. The total blood volume increases by about 40% above nonpregnant levels, with wide individual variations. The plasma volume rises as early as the 6th week of pregnancy and reaches a plateau by about 32 to 34 weeks’ gestation, after which little further change occurs. The increase averages 50% in singleton pregnancies and approaches 70% with a twin gestation. The red blood cell mass begins to increase at the start of the second trimester and continues to rise throughout pregnancy. By the time of delivery, it is 20% to 35% above nonpregnant levels. The disproportionate increase in plasma volume compared with the red cell volume results in hemodilution with a decreased hematocrit reading, sometimes referred to as physiologic anemia of pregnancy. If iron stores are adequate, the hematocrit tends to rise from the second to the third trimester.
TABLE 6-2 CARDIOVASCULAR CHANGES IN PREGNANCY
| Parameter | Amount of Change | Timing |
|---|---|---|
| Arterial blood pressures | ||
| Systolic | ↓ 4-6 mm Hg | All bottom at 20-24 wk, then rise gradually to prepregnancy values at term |
| Diastolic | ↓ 8-15 mm Hg | |
| Mean | ↓ 6-10 mm Hg | |
| Heart rate | ↑ 12-18 beats/min | 1st, 2nd, 3rd trimesters |
| Stroke volume | ↑ 10%-30% | 1st and 2nd trimesters, then stable until term |
| Cardiac output | ↑ 33%-45% | Peaks in early 2nd trimester, then stable until term |
Data from Main DM, Main EK: Obstetrics and Gynecology: A Pocket Reference. Chicago, Year Book, 1984, p 18.
 Respiratory System
Respiratory System
RESPIRATORY MECHANICS IN PREGNANCY
The changes in lung volume and capacities associated with pregnancy are detailed in Table 6-3. Assessment of mechanical changes during pregnancy reveals that the diaphragm at rest rises to a level of 4 cm above its usual resting position. The chest enlarges in transverse diameter by about 2.1 cm. Simultaneously, the subcostal angle increases from an average of 68.5 degrees to 103.5 degrees during the latter part of gestation. The increase in uterine size cannot completely explain the changes in chest configuration because these mechanical changes occur early in gestation.
TABLE 6-3 LUNG VOLUMES AND CAPACITIES IN PREGNANCY
| Test | Definition | Change in Pregnancy |
|---|---|---|
| Respiratory rate | Breaths/minute | No significant change |
| Tidal volume | The volume of air inspired and expired at each breath | Progressive rise throughout pregnancy of 0.1-0.2 L |
| Expiratory reserve volume | The maximum volume of air that can be additionally expired after a normal expiration | Lowered by about 15% (0.55 L in late pregnancy compared with 0.65 L postpartum) |
| Residual volume | The volume of air remaining in the lungs after a maximum expiration | Falls considerably (0.77 L in late pregnancy compared with 0.96 L postpartum) |
| Vital capacity | The maximum volume of air that can be forcibly inspired after a maximum expiration | Unchanged, except for possibly a small terminal diminution |
| Inspiratory capacity | The maximum volume of air that can be inspired from resting expiratory level | Increased by about 5% |
| Functional residual capacity | The volume of air in lungs at resting expiratory level | Lowered by about 18% |
| Minute ventilation | The volume of air inspired or expired in 1 min | Increased by about 40% as a result of the increased tidal volume and unchanged respiratory rate |
Data from Main DM, Main EK: Obstetrics and Gynecology: A Pocket Reference. Chicago, Year Book, 1984, p 14.
 Renal Physiology
Renal Physiology


