CHAPTER 42 Malignant disease of the uterus
Introduction
Worldwide, approximately 200,000 women are diagnosed with endometrial cancer each year (Ferlay et al 2004). Of these, more than two-thirds arise in developed countries where age-standardized incidence is four times higher than in developing countries (Ferlay et al 2007). Endometrial cancer is now the most common gynaecological malignancy in Western Europe and North America, with an annual incidence of 81,500 women in the European Union and 40,100 women in North America. In the UK, the incidence of endometrial cancer increased by 29.3% between 1993 and 2005, with an incidence of 6891 women in 2005 (Office for National Statistics 2008). This increase is attributable to an increase in obesity, tamoxifen use for breast cancer and increased life expectancy (Reich 1992, Kitchener 2008). Given the anticipated increase in obesity, it is likely that this trend will continue.
Endometrial cancer has traditionally been believed to have a relatively good outcome. Whilst commonly believed to be a curable cancer in contrast to other gynaecological malignancies, the overall 5-year survival rate is disappointing considering that 75% of patients present with early (stage I) disease. Relative survival at both 1 and 5 years after diagnosis has risen steadily from 85% and 72%, respectively, in the late 1980s to 88% and 76% in the late 1990s (Cooper et al 2008). However, there is a significant gap in survival between the most affluent and the most deprived groups of approximately 4% based on data from England and Wales, with survival lagging 2–6% behind Europe (Sant et al 2003, Cooper et al 2008). This deprivation gap may be best explained by comorbidities such as obesity, hypertension and cardiovascular disease, which are more common in deprived communities (Kitchener 2008). In addition, survival in advanced-stage endometrial cancer and poor prognostic types of endometrial cancer continues to be poor.
With the implementation of improving outcomes guidance in endometrial cancer, women with higher grade and poor prognostic types of endometrial cancer should be managed in gynaecological oncology centres, thus centralizing resources and experience, and facilitating trials that investigate new therapies for these patients (Department of Health 1999).
Epidemiology
Most studies focus on the epidemiology of endometrioid cancers, as the epidemiology for non-endometrioid histotypes is less clear (Box 42.1).
Obesity
Body mass index (BMI) plays an important role with a strong linear increase in risk with increasing BMI. There is a six-fold increase in women with a BMI greater than 40 kg/m2 compared with those with a normal BMI (20–24 kg/m2), whereas women with a BMI under 20 kg/m2 have half the risk (Lindemann et al 2008). The fact that obesity increases risk has been attributed to changes in concentrations of endogenous hormones in obese women. Oestrogens produced in adipose tissue have a direct mitogenic effect on endometrial cells. In postmenopausal women, oestrogens derived from peripheral adipose tissue are the primary source of endogenous oestrogen, and the rate of production is related to the size of the adipose depots. In younger premenopausal women, obesity can also be associated with anovulatory cycles, resulting in the absence of counterbalancing progesterone. Oligo- and amenorrhoeic women with polycystic ovary syndrome can develop endometrial hyperplasia and later carcinoma. There is a weight-related increase in insulin and insulin-like growth factor-I, both of which are endometrial growth factors. Cytokines produced in fat tissue (leptin and adiponectin) may play a direct role in endometrial carcinogenesis, as well as transcription factors that can modulate both cellular lipid metabolism and tumorigenesis. There is also a strongly increased risk of endometrial cancer in diabetics, possibly because of shared underlying mechanisms.
Tamoxifen
Tamoxifen improves the survival of postmenopausal women with hormone-receptor-positive breast cancer. Breast and endometrial cancer share several risk factors including age of menopause, unopposed oestrogen use and obesity, and breast cancer patients have an increased rate of endometrial cancer regardless of endocrine therapy. The risk of endometrial cancer rises with both the use of higher doses and increasing duration of tamoxifen use. Treatment beyond 5 years increases the risk at least four fold. However, the absolute risk of endometrial cancer is still very small in tamoxifen-treated women (Early Breast Cancer Trialists’ Collaborative Group 1998). The use of aromatase inhibitors for postoperative adjuvant therapy instead of tamoxifen significantly reduces the risk of adverse gynaecological events (Duffy et al 2009). Switching from tamoxifen to an aromatase inhibitor may reduce the risk of endometrial cancer, but needs to be balanced against the side-effects and costs of therapy.
Hereditary non-polyposis colorectal cancer
Hereditary non-polyposis colorectal cancer (HNPCC) is one of the most common cancer family syndromes. The estimated lifetime risk of developing endometrial cancer in women carrying these mutations is approximately 42–60%. HNPCC results from germline mutations in DNA mismatch repair genes and is autosomal dominant in inheritance (Froggatt et al 1996). As well as an increased risk of malignancies in the colon, cancers of the endometrium, ovary, biliary tract, stomach, small intestine and renal tract occur more commonly in these women (Box 42.2). Endometrial cancer is the most common and usually occurs in the premenopausal period. The majority of mismatch repair gene defects involve the genes MSH2 and MLH1, with defects in PMS1 and PMS2 being less common (Papadopoulos and Lindblom 1997). A good family history will help to identify women who may carry these mutations. If such a mutation is found or if the woman is thought to be at high risk, there may be a role for prophylactic hysterectomy and bilateral salpingo-oophorectomy. Screening may be offered to those who do not wish to have surgery but with the caveat that there is no evidence that it is effective. There may also be a role for prophylactic progesterone in these women, and studies are currently investigating the role of the Mirena™ intrauterine system for prophylaxis.
Box 42.2 Lifetime risk of malignancy in women with HNPCC
| Colorectal | 30–50% |
| Endometrial | 40–60% |
| Ovarian | 9–12% |
| Gastric | 2–7% |
| Biliary | 13–15% |
| Urinary | 4–5% |
Hormones
The combined oral contraceptive pill has a large protective effect that lasts for at least 20 years after cessation. Older hormone replacement therapy (HRT) regimens that utilized unopposed oestrogen in women with an intact uterus increase the relative risk of endometrial carcinoma approximately six fold after 5 years of use (Weiderpass et al 1999). It is strongly recommended that women with intact uteri should not be started on regimes containing oestrogen alone, and should be offered endometrial protection by regimes with either 10–12 days of cyclical progestogens or continuous combined regimens (Lethaby et al 2004).
Pathology
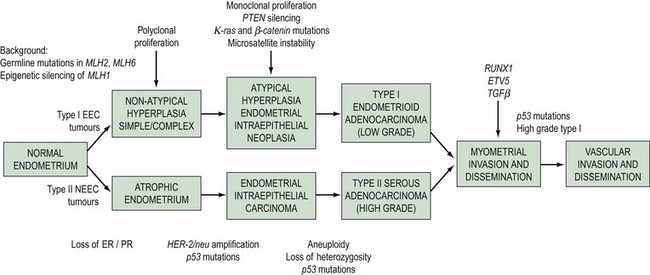
There is a growing consensus that endometrial cancers can be classified into two types based on clinical and molecular characteristics. Type I endometrial cancers (80%) are oestrogen dependent, usually arise in a background of endometrial hyperplasia, have well to moderately differentiated endometrioid histology, tend to be biologically indolent and have a good prognosis (Bokhman 1983). Type II cancers are not oestrogen driven, have a higher grade, occur in a background of atrophic endometrium and have a poorer prognosis (Bokhman 1983). Uterine papillary serous carcinomas and clear cell carcinomas are considered to be type II endometrial cancers. These cancers, and uterine serous papillary cancer in particular, are characterized by early metastasis, resistance to therapy and a high mortality rate. Within the type II cancer histological subtypes, Creasman et al (2004) found 5-year survival of 81% for surgically staged stage I clear cell cancer and 72% for uterine papillary serous cancer. This is in comparison with over 95% for well to moderately differentiated stage IA endometrioid cancers. Although unusual cancers only account for 10% of endometrial cancers, they account for more than 50% of recurrences and deaths (Acharya et al 2005).
The morphological and clinical differences are paralleled by genetic distinctions (Hecht and Mutter 2006). Type I cancers are associated with mutations in the K-ras2 oncogene, loss of the PTEN tumour suppressor gene, defects in DNA mismatch repair and near-diploid karyotype, whereas type II cancers are associated with mutations in TP 53 and ERBB-2 expression, and most are non-diploid. A dualistic model of endometrial tumorigenesis has been proposed which could explain the very different clinical outcomes in these groups of patients (Table 42.1, Figure 42.1). An understanding of the molecular heterogeneity of various histological types of endometrial cancer has the potential to lead to better individualization of treatment in the future (Maxwell et al 2005).
Table 42.1 Clinicopathological characteristics and genetic abnormalities in type I and type II endometrial carcinomas
| Characteristic | Type I | Type II |
|---|---|---|
| Unopposed oestrogen | Yes | No |
| Background endometrium | Hyperplastic | Atrophic |
| Morphology | Endometrioid | Serous, clear cell |
| Microsatellite instability | 20–40% | 0–5% |
| p53 mutations | 10–20% | 90% |
| β-Catenin mutations | 31–47% | 0–3% |
| K-ras mutations | 15–30% | 0–5% |
| PTEN inactivation | 35–50% | 10% |
| HER-2/neu | No information | 18–80% |
Source: From Doll A, Abal M, Rigau M, Monge M, Gonzalez M, Demajo S, et al. Novel molecular profiles of endometrial cancer-new light through old windows. J Steroid Biochem Mol Biol 2008;108(3–5):221–9.
Histopathology
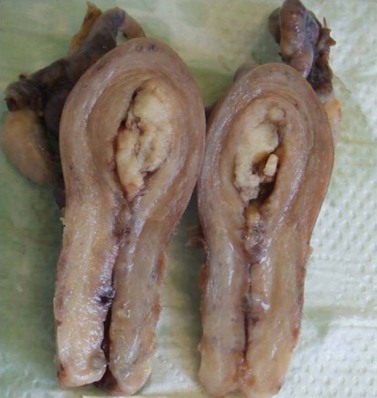
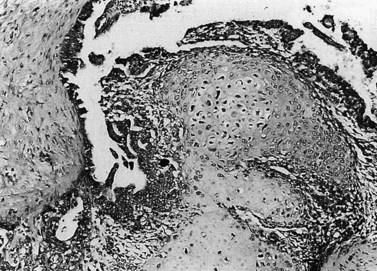
Endometrial carcinoma is usually seen as a raised, rough, perhaps papillary area occupying at least half of the endometrium. It often arises in the fundal region of the uterus; the internal os is rarely involved early in the disease (Figure 42.2). Myometrial invasion may be obvious to the naked eye. There seems to be no correlation between the degree of exophytic growth of the tumour within the uterine cavity and the presence of myometrial invasion.
There are several different histological subtypes of endometrial carcinoma (Table 42.2).
Table 42.2 Classification of endometrial carcinomas
| Endometrioid (usual) carcinoma (includes glandular and villoglandular patterns) |
| Secretory |
| Ciliated cell |
| With squamous differentiation (includes adenoacanthoma and adenosquamous carcinoma) |
| Special variant carcinomas |
| Papillary serous adenocarcinoma |
| Clear cell carcinoma |
| Mucinous carcinoma |
| Pure squamous cell carcinoma |
| Mixed |
| Undifferentiated |
| Carcinosarcoma |
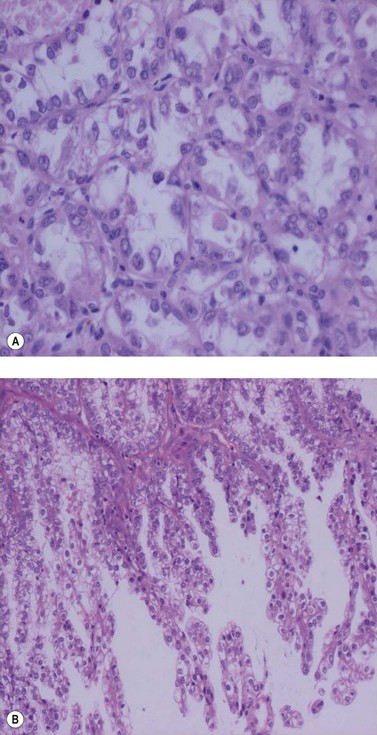
Endometrioid adenocarcinoma
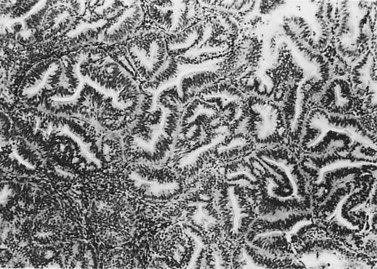
The most common endometrial carcinoma is referred to as endometrioid adenocarcinoma, the glandular pattern of which generally resembles that of normal proliferative-phase endometrium, although sometimes showing extreme complexity of the glands and cribriform pattern (Figure 42.3). Multilayering of the epithelial cells is nearly always seen. The secretory and ciliated cell variants are rare and well differentiated by definition. Secretory adenocarcinoma is a well-differentiated adenocarcinoma characterized by neoplastic glands with a subnuclear vacuolization resembling normal 17-day secretory endometrium. Mucinous adenocarcinoma is a low-grade neoplasm in which most of the cells contain prominent intracytoplasmic mucin, and should be distinguished from otherwise typical endometrioid carcinomas with abundant extracellular mucin but no intracellular mucin.
Endometrial adenocarcinoma with squamous metaplasia (adenoacanthoma)
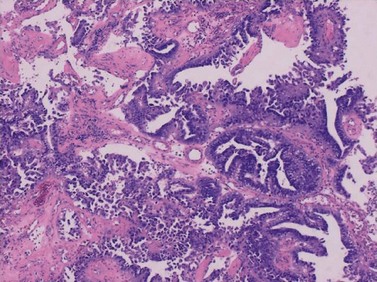
Up to 25% of endometrioid-pattern endometrial adenocarcinomas contain areas of squamous metaplasia. Those tumours in which the squamous component is morphologically benign are commonly termed ‘adenoacanthomas’. The squamous change is seen as islands of typical squamous epithelium, very often situated within the gland lumina or as surface or diffuse squamous metaplasia (Figure 42.4).
Papillary serous and clear cell adenocarcinomas
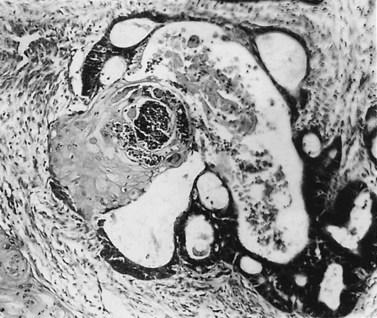
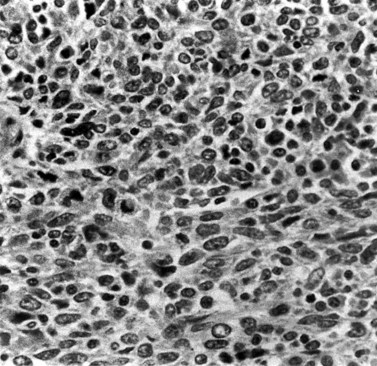
These are less common histological subtypes, both associated with a poor prognosis. Clear cell carcinoma is composed of large, clear cells containing glycogen, often forming papillary structures lined by ‘hobnail’ cells (Figure 42.5). Papillary serous carcinoma closely resembles ovarian papillary serous carcinoma, featuring severe atypia, a complex papillary pattern, high mitotic index, prominent myometrial invasion and psammoma bodies (Figure 42.6). Most patients are postmenopausal.
Histopathological reporting
It is essential not only to recognize specific subtypes and to grade carcinomas accurately, but also to record the extent of myometrial invasion. The maximum depth of myometrial invasion, overall thickness of the myometrium, and minimum distance of the tumour from the serosal surface should be recorded. The presence of lymphovascular invasion should be noted. The cervical stroma, fallopian tubes and ovaries should all be examined carefully for tumour. Histopathological examination of the omentum would be valuable in uterine papillary serous carcinoma and clear cell carcinoma. The number of lymph nodes removed, the number of lymph nodes positive for malignancy, extracapsular spread and the extent of lymphatic spread (e.g. node replaced with malignancy or not) should also be reported. There is no convincing evidence for routine ultrastaging or special immunohistochemical analysis of surgically removed lymph nodes (Figure 42.7).
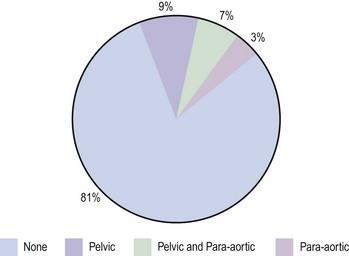
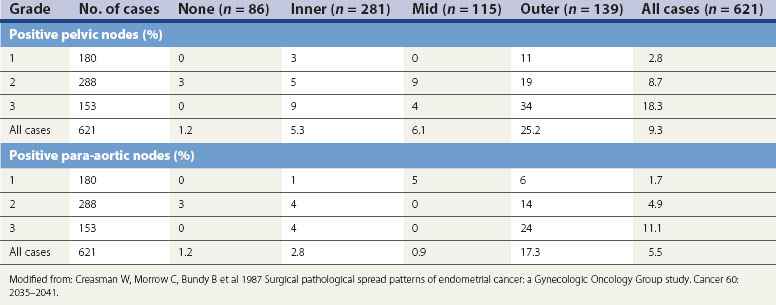
Figure 42.7 Lymph node involvement in clinical stage I endometrial carcinoma
Source: Ayhan et al 1994 Surgical pathological spread patterns of endometrial cancer: A Gynecologic Oncology Group Study. Cancer 60: 2035–2041.
The national minimum dataset published by the Royal College of Pathologists provides a useful tool for both gynaecologists and histopathologists. Serous carcinoma, clear cell carcinoma, and squamous and undifferentiated carcinoma are not graded, these tumours being highly malignant neoplasia. Sarcomas are also highly malignant neoplasms (Figures 42.8–42.11). The architectural grade, nuclear grade and FIGO grade of tumours can be used to separate patients into groups with significantly different rates of progression of disease and relative survival.
Endometrial hyperplasia
Simple glandular hyperplasia is no longer considered to be a premalignant condition, but atypical hyperplasia is an ominous finding. Approximately 10–20% of all cases with atypia will have an underlying carcinoma present, and invasive cancer will develop later in up to 50% of cases. These figures are higher in the case of complex atypical hyperplasia than simple atypical hyperplasia (Silverberg 2000). Women with hyperplasia without cytological atypia may be treated with cyclical or continuous progestogens for 6 months and then undergo rebiopsy. Women with lesions showing cytological atypia who have finished childbearing should be offered a hysterectomy. The management of younger women with atypical hyperplasia is more difficult. Hyperplasia is discussed further in Chapter 38.
Spread of Disease
The current FIGO staging criteria are shown in Box 42.3.
Box 42.3
FIGO surgical staging of endometrial carcinoma
| Stage Ia | Tumor confined to the corpus uteri |
| IAa | No or less than half myometrial invasion |
| IBa | Invasion equal to or more than half of the myometrium |
| Stage IIa | Tumor invades cervical stroma, but does not extend beyond the uterusb |
| Stage IIIa | Local and/or regional spread of the tumor |
| IIIAa | Tumor invades the serosa of the corpus uteri and/or adnexaec |
| IIIBa | Vaginal and/or parametrial involvementc |
| IIICa | Metastases to pelvic and/or para-aortic lymph nodesc |
| IIIC1a | Positive pelvic nodes |
| IIIC2a | Positive para-aortic lymph nodes with or without positive pelvic lymph nodes |
| Stage IVa | Tumor invades bladder and/or bowel mucosa, and/or distant metastases |
| IVAa | Tumor invasion of bladder and/or bowel mucosa |
| IVBa | Distant metastases, including intra-abdominal metastases and/or inguinal lymph nodes |
b Endocervical glandular involvement only should be considered as Stage I and no longer as Stage II.
c Positive cytology has to be reported separately without changing the stage.
Source: FIGO Committee on Gynecologic Oncology 2009 Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. International Journal of Gynecology and Obstetrics 105: 103–104.
FIGO Staging
Depth of myometrial penetration is a more important guide to the likelihood of nodal metastases than tumour grade (Table 42.3; Creasman et al 1987). Serous papillary and clear cell cancers have the highest risk of spread to para-aortic nodes. Only 10% of cases subsequently proven to have nodal spread had clinically enlarged nodes but, on the other hand, enlarged lymph nodes are not necessarily involved with metastatic disease. Serous papillary carcinoma of the endometrium, histologically similar to serous papillary cancer of the ovary, has a very poor prognosis and a pattern of spread akin to ovarian malignancy.
Positive peritoneal washings are present in approximately 12–15% of all cases of endometrial cancer, with half of these having no histological evidence of extrauterine spread. The Gynaecological Cancer Intergroup have proposed changes to the FIGO staging which have been incorporated into the revised FIGO staging for endometrial cancer (Box 42.3). They recommend that the grading of tumours is added to stage I tumours as this carries a direct clinical relevance. They also recommend removing the upgrading to stage IIIC on the basis of positive peritoneal washings, as these are very likely due to handling at the time of surgery and do not have independent prognostic significance; and that metastasis to positive inguinal lymph nodes is considered stage IIIC rather than stage IV. Although not part of FIGO staging, some centres perform a serum CA125 in women with endometrial cancer as reports suggest that an elevated serum CA125 at diagnosis indicates a high risk of subsequent recurrence but is less effective at predicting myometrial invasion than ultrasound.