CHAPTER 47 Malignant disease of the breast
Introduction
Breast cancer is one of the most common malignancies afflicting women, and is the leading cause of cancer-related mortality (Hortobagyi et al 2005). Worldwide, more than 1.2 million women are diagnosed with breast cancer each year, affecting 10–12% of the female population and resulting in 500,000 deaths per year. In the UK in 2005, there were 45,947 new cases: 45,660 (over 99%) in women and 287 (<1%) in men, causing approximately 14,000 deaths per year (Information Statistics Division Online 2008, Office for National Statistics 2008, Northern Ireland Cancer Registry 2008, Welsh Cancer Intelligence and Surveillance Unit 2008). The lifetime risk of a women being diagnosed with breast cancer is one in nine (Health Statistics Quarterly 1999, Information Statistics Division Online 2008, Welsh Cancer Intelligence and Surveillance Unit 2008). Whilst it is impossible to predict who will develop breast cancer, it is possible to identify those women who are at increased risk for breast cancer and provide options for reducing the risk.
Trends in Breast Cancer Incidence and Prevalance
More than 80% of cases of breast cancer occur in women over 50 years of age, with the highest incidence in women aged 50–69 years. The incidence of breast cancer has been increasing for many years in economically developed countries. The UK age-standardized incidence of breast cancer per 100,000 women increased from 74 in 1975 to 123 in 2005 (Coleman 2000). The introduction of a national screening programme in the UK in 1988 led to a transient increase in breast cancer incidence in women aged 50–64 years, as early undiagnosed cancers were detected. This increase lasted for the first 4–7 years of the programme. However, an underlying increase in incidence predating screening continues today and is particularly evident in older age groups. Mortality rates for breast cancer have fallen in many developed countries since 1990, having been previously stable/increasing for several decades (Beral et al 1995, Peto et al 2000, Jatoi and Miller 2003). This reduction has been attributed to earlier detection following the implementation of breast screening, decreased use of exogenous hormones and the use of adjuvant therapies such as tamoxifen (Berry et al 2005).
As the incidence of breast cancer is high and the 5-year survival rate is approximately 80%, many women are alive who have been diagnosed with breast cancer. An estimated 550,000 women are alive in the UK who have received a diagnosis of breast cancer (Maddams et al 2008).
Risk Factors
Age
Age is the single most important risk factor for the development of breast cancer (Colditz and Rosner 2000). The incidence of breast cancer increases with age, doubling every 10 years to the menopause, and then the rate of increase slows, levelling to a plateau after 80 years (Anderson et al 2005). This creates a point of inflection in the age-specific incidence curve known as ‘Clemmensen’s hook’ (Clemmensen 1948). The incidence of oestrogen-receptor-alpha (ERα)-negative tumours rises rapidly to 50 years and then flattens out/decreases, whereas the incidence of ERα-positive tumours is similar up to the age of 50 years but then continues to increase, albeit at a slower pace. As a result, ERα-negative tumours tend to occur earlier in life and ERα-positive tumours are more common in older women. The peak age of onset for these two tumour phenotypes is 50 years and 70 years, respectively.
Ethnicity changes the effect of age on breast cancer risk. African-American women under 50 years of age have a higher age-specific incidence of breast cancer than their Caucasian counterparts (Vogel 1998). However, the incidence of breast cancer is higher in Caucasian women after 50 years of age. The difference in age-adjusted breast cancer mortality rates between African-American and Caucasian women in the 1980s was probably due to the greater incidence of ERα-negative tumours in African-American women, who will not have benefited from the introduction of adjuvant hormonal therapy (Jatoi et al 2005).
Geographical variation
Worldwide, more than 1 million women are diagnosed with breast cancer every year, accounting for 10% of all cancer cases and 23% of all female cancer cases (Ferlay et al 2004). Incidence rates for breast cancer are six times greater in the Western world than in underdeveloped countries. Approximately 430,000 new cases of breast cancer occur each year in Europe and an estimated 212,920 in the USA. The lowest rates in Europe are found in Romania and Latvia, and the highest rates are found in Northern and Western Europe. The incidence of breast cancer in American Hispanic women is 40–50% that of non-Hispanic White women.
Migrants from low-risk countries to high-risk countries have been shown to acquire the risk of the ‘host nation’ within two generations (Tominaga 1985, Ziegler et al 1993). Japanese migrants to the USA acquire an increased breast cancer risk compared with the population in Japan, and there is increasing evidence that the earlier in life a woman takes up residence in a ‘high-risk’ country, the higher the risk of breast cancer compared with the country of origin (Shimizu et al 1991, Ziegler et al 1993).
Ovulatory cycles
Events in a woman’s life that alter her lifetime ovulatory cycles appear to correlate with the risk of breast cancer. Women who start menstruating early or have a late menopause have a 30–50% increase in risk of developing breast cancer. Similarly, late menarche and an early menopause lead to an equivalent reduction in breast cancer risk. The risk of developing breast cancer is doubled in those women who have a natural menopause after 55 years of age compared with those who experience the menopause before 45 years of age. A menopause induced before 40 years of age reduces the risk of breast cancer by almost two-thirds (Hankinson et al 2004).
Age at first pregnancy
Late age at first birth and nulliparity increase the lifetime risk of breast cancer. Nulliparity has been a well-known risk factor for developing breast cancer since Ramizzini described horrendis mammarium canceris in Catholic nuns (Ramazzini 1713).The risk of breast cancer in women who have their first child after 30 years of age is double that of women who have their first child before 20 years of age. The group at highest risk are those women who have their first child after 35 years of age. These women have a greater risk than nulliparous women. An early age of birth of a second child confers a reduced risk. The protective effects of an early full-term pregnancy have been observed in a number of ethnic groups and geographic locations, suggesting that the parity-induced protection results from biological changes in the breast rather than environmental factors.
Benign breast disease
Prospective and retrospective studies have shown a relative risk of breast cancer of 1.5–1.6 for women with benign breast disease compared with the general population (Hartmann et al 2005). This increased risk has been shown to persist for 25 years after biopsy. The relative risk of proliferative changes with atypia was 4.24 compared with a relative risk of 1.88 for those without atypia and 1.27 for non-proliferative lesions. The age at diagnosis of the benign breast disease appears to modify the risks related to the histological appearance of benign breast disease. The presence of atypia in women under 45 years of age conveys twice the risk observed among women over 55 years of age. The Breast Cancer Detection and Demonstration Project showed that the risk of breast cancer among premenopausal women with atypia was elevated by a factor of 12.0, compared with 3.3 for postmenopausal women with aytpia (London et al 1992, Dupont et al 1993). An increase in breast cancers has been demonstrated in the same breast during the first 5 years of follow-up following a diagnosis of benign breast disease, particularly in women with atypia.
Ionizing radiation exposure
The understanding of radiation-related breast cancer in women derives from epidemiological studies of patients exposed to diagnostic or therapeutic medical radiation (mantle irradiation for lymphoma) and of the Japanese atomic bomb survivors (United Nations Scientific Committee on the Effects of Atomic Radiation 2000). The breast tissue of young women is highly sensitive to the carcinogenic action of ionizing radiation. Only the bone marrow and the infant thyroid gland are more sensitive to the cancer-causing effects of radiation. Ionizing radiation is an established breast cancer risk factor and this risk has been shown to increase linearly with dose. Age at exposure directly affects radiation-related breast cancer risk, with the greatest risk seen in women exposed before 20 years of age and a significantly reduced risk seen for postmenopausal women. Periods of enhanced cell proliferation, namely in utero, puberty and pregnancy, have been proposed to represent windows of increased susceptibility for mammary carcinogenesis (Ronckers et al 2005).
Oral contraceptive pill
Use of the oral contraceptive pill slightly increases the risk of breast cancer in current and recent users (1–4 years following cessation) with relative risks of 1.24 and 1.16, respectively (Collaborative Group on Hormonal Factors in Breast Cancer 2002). This risk diminishes after discontinuing use and returns to normal after 10 years.
These estimates are based on the Collaborative Group on Hormonal Factors in Breast Cancer study; a collaborative meta-analysis of 54 studies in 25 countries with data on over 50,000 women with breast cancer (Anonymous 1996). Cancers diagnosed in women who have used the oral contraceptive pill tend to be less clinically advanced than those detected in women who have never used it. Users of the oral contraceptive pill are generally younger women whose breast cancer risk is comparatively low, so the small excess in current users will result in a relatively small number of additional cases. Other findings of the study were:
A large case–controlled study of 4575 women aged 35–64 years showed that current or former use of the oral contraceptive pill was not associated with a significantly increased risk of breast cancer (Marchbanks et al 2002).
Hormone replacement therapy
Hormone replacement therapy (HRT) use increases the risk of breast cancer and reduces the sensitivity of mammography. The risk of breast cancer for current or recent users of HRT increases by 2.3% per year of use. For women who have used HRT for at least 5 years (average 11 years), the increased risk was 35% (Anonymous 1997a). The effect is substantially greater for oestrogen–progesterone combinations than for oestrogen-only HRT. Risk increases with duration of use; the risk for current users of oestrogen–progesterone combinations for more than 10 years was 2.31 compared with 1.74 for 1–4 years of use. Risk decreases with cessation of use; past users (>5 years following cessation) have a similar risk to those who have never taken HRT. One recent study reported that current users of combined HRT had a 2.7-fold elevated risk of lobular cancer and a 3.3-fold risk of ductal cancer. The risk of lobular cancer was only raised in women who had used HRT for 3 years or more.
In the UK over the past 10 years, it is estimated that 20,000 extra breast cancer cases have occurred among women aged 50–64 years as a result of HRT use, and three-quarters (15,000) of these additional breast cancers are due to the use of oestrogen–progesterone HRT. HRT is used by over 20 million women in Western countries for the alleviation of perimenopausal and postmenopausal symptoms, and is an important source of exogenous oestrogen and, in some cases, progesterone exposure. A recent review concluded that the excess incidence of breast cancer, stroke and pulmonary embolism in postmenopausal women who use HRT for 5 years was greater than the reduction in incidence of colorectal cancer and hip fracture (Beral et al 2002). The risks and benefits for treating menopausal symptoms should be evaluated on an individual basis.
A decrease in the incidence of breast cancer among postmenopausal women in the UK may be linked to a decrease in the use of HRT. Researchers calculated that between 1999 and 2005, the risk of the disease fell by 14% in women in their 50s, representing 1400 fewer cases in 2005, and 3300 fewer over the period (Parkin 2009). They suggest that the decrease in breast cancer since 1999 in women aged 50–59 years and since 2003 in women aged 60–64 years is a consequence of the reduced use of HRT. The use of HRT began to fall after the Million Women Study in the UK and the Women’s Health Initiative Study in the USA showed that HRT could increase the risk of breast cancer. In women aged 45–69 years, the use of HRT increased from 1992 to reach a peak of 25% in 2000–2001, before falling to approximately half that by 2006.
Hormone therapy after breast cancer is becoming an increasingly relevant problem as more women survive breast cancer. The HABITS (Hormonal Replacement Therapy After Breast Cancer — Is It Safe?) trial was designed to confirm the efficacy of HRT given to women after treatment for breast cancer (Holmberg and Anderson 2004). The trial confirmed an unacceptably high risk in women allocated to receive HRT compared with those receiving best symptomatic treatment, and as a result was terminated. Analysis of women in the HABITS trial after a median follow-up of 4 years showed that 17.6% in the HRT treatment arm had developed breast cancer recurrence or a new breast cancer, compared with 7.7% in the control arm. The estimated 5-year cumulative rate for disease recurrence was 22.2% in the HRT arm and 9.5% in the control arm.
Endogenous hormones
Higher levels of endogenous hormones have been hypothesized to increase breast cancer risk. A pooled analysis of nine prospective cohort studies found a statistically significant increased risk of breast cancer in postmenopausal women with higher levels of sex hormones (Key et al 2002). The risk for women whose oestradiol levels were in the top quintile was approximately twice that compared with women whose oestradiol levels were in the bottom quintile. Evidence in premenopausal women was inconclusive.
Diet
A high-fat diet has been positively associated with breast cancer in a number of animal and case–control studies. Pooled analysis of cohort studies found no significant association between fat intake and breast cancer risk, whilst a meta-analysis found an association between higher total and saturated fat intake and an increased risk of breast cancer. A recent prospective study confirmed a significant association between saturated fat and breast cancer risk (Bingham et al 2003).
Alcohol intake
A significant association between alcohol intake and breast cancer has been found, with an increased risk of 7% for each 10 g alcohol/day (Hamajima et al 2002, Baan et al 2007). Approximately 4% of breast cancers in women from developed countries may be attributable to alcohol. In a recent large cohort of middle-aged women in the UK, a population with low-to-moderate alcohol consumption [10 g alcohol (one drink)/day] has shown a statistically increased risk of breast cancer; they also showed an increase in cancers of the oral cavity, pharynx, oesophagus, larynx, rectum and liver (Allen et al 2009). They calculated that the increased risk accounted for an additional 11 breast cancers per 1000 women up to 75 years of age. Although alcohol and tobacco are closely related social habits, there is no direct association between tobacco and breast cancer.
Height
Taller women have an increased risk of breast cancer (Hunter and Willett 1993). A pooled analysis estimated that the relative risk for women over 1.75 m tall compared with women under 1.6 m tall was 1.22 for all women and 1.28 for postmenopausal women (van den Brandt et al 2000).
Physical activity
A report from the International Agency for Research on Cancer concluded that physical activity has a preventive effect on breast cancer. This may be an indirect effect as exercise lowers BMI, or a direct effect on hormonal and growth factor levels. This varies between studies, with one showing a 30–40% reduction in the risk of breast cancer with a few hours of vigorous activity per week versus none (Monninkhof et al 2007).
Mammographic density
There is extensive evidence that mammographic density is a risk factor for breast cancer, independent of other risk factors, and is associated with large relative and attributable risks for the disease. Women with increasingly dense breasts have two to six times the risk of breast cancer compared with women with less dense breasts (Boyd et al 1995). Parity, menopause and other risk factors only explain 20–30% of the variance in mammographic density (Boyd et al 2002). Early studies of mother–daughter sets and small twin studies suggested that genetic factors might explain a proportion of the variation of breast tissue patterns within a given population (Boyd et al 2002, Stone et al 2006). The genetic factors that influence mammographic density may also explain variations in breast tissue involution.
Previous history of breast cancer
If a woman has had breast cancer previously, the risk of developing a second primary breast cancer is two to six times greater compared with the the risk of the general population of developing a primary breast cancer (Chen and Thompson 1999).
Family history
A family history of breast cancer, particularly in first-degree relatives, is a well-known risk factor for the development of breast cancer. The past few years have seen a significant increase in knowledge of inherited breast cancer. Approximately 80% of breast cancers are sporadic, and the other 20% are familial, occurring within the context of a positive family history. Twin studies have shown that inherited factors may account for up to 25–30% of all breast cancers (Lalloo et al 2003). These cancers are the result of a combination of genetic and environmental factors that cause acquired genetic mutations over time (Vogel and Bevers 2003). A woman with one first-degree relative has approximately double the risk of breast cancer of a woman with no family history of the disease. The risk is greater if two (or more) relatives are affected. Multiple primary cancers in one individual or related early-onset cancers in a family pedigree are highly suggestive of a predisposing gene.
BRCA1 and BRCA2 account for the largest proportion of familial breast cancer cases. It is thought that 20–25% of familial breast cancer cases are due to mutations or genomic rearrangements within these genes. The frequency of these mutations is rare, occurring in 0.1–0.5% of the general population, compared with 2% in Ashkenazi Jews (Rebbeck et al 2002). The breast cancer risk attributable to BRCA1 and BRCA2 mutations in Ashkenazi Jews is as high as 15–30% (FitzGerald et al 1996, Abeliovich et al 1997, Struewing et al 1997, Metcalfe et al 2004). A recent analysis of 22 studies showed that carrying a deleterious BRCA1 or BRCA2 mutation confers an estimated lifetime risk for developing breast cancer of 65% and 45%, respectively (Antoniou et al 2003). By the age of 40 years, carrying a deleterious BRCA1 mutation confers a 20% chance of developing breast cancer, and the risk increases with age (Kauff et al 2002). Mutations in BRCA1 are strongly associated with ovarian and fallopian tube cancers (Antoniou et al 2003). The risk of ovarian carcinoma for BRCA1 carriers is 17%, 39% and 54% at 40, 70 and 80 years, respectively (Antoniou et al 2003). Penetrance of BRCA1 with respect to ovarian, fallopian and breast cancer is greater than that for BRCA2.
Tumours associated with BRCA1 carriers are more frequently grade 3, and ERα-negative and ovarian carcinomas, which occur in BRCA1/2 families, are mostly non-mucinous epithelial cancers (Lakhani et al 2002). No single technique is able to detect all mutations, and even by sequencing the entire gene, the detection rate is only 85% (Evans et al 2003). Once a mutation has been identified in a family, definitive genetic testing can inform women more accurately of their risks and give them an informed choice of their options. Mutational analysis of an unaffected individual is problematic without checking an affected relative. Identification of a mutation will confirm an increased risk; however, the absence of a mutation does not exclude the possibility of a refractory mutation of another gene. Where there is not a dominant family history or a BRCA1/2 mutation is not identified, risk estimation gives a 1.5–3 fold relative risk with a family history of a single first-degree relative affected (Newman et al 1988, Claus et al 1994).
Quantitative Risk Assessment
The Gail model
The Gail model quantifies a woman’s lifetime risk of developing breast cancer by incorporating patient age, age at menarche, age at first birth/nulliparity, number of breast biopsies, ethnic origin and history of breast cancer in first-degree relatives (Gail et al 1989, Costantino et al 1999). It does not, however, consider the ages of first-degree relatives with breast cancer, and overlooks a family history of bilateral breast disease, second-degree relatives with breast cancer and a family history of ovarian cancer. Also, the model does not account for a personal history of atypical hyperplasia and lobular carcinoma in situ, previous radiation therapy to the chest for the treatment of Hodgkin’s lymphoma, or recent migration from a region of low breast cancer risk (e.g. rural China) to a high-risk region. The Gail model remains the most frequently used in defining eligibility for risk reduction trials.
The Claus model
The Claus model provides breast cancer risk estimates based on which relatives were diagnosed with breast cancer and at what age their diagnosis was made. Initially, only data from mothers and sisters were included, but second-degree relatives were included subsequently (Claus et al 1994).
BRCAPRO
BRCAPRO was developed by statisticians at Duke University to calculate age-specific probabilities of developing breast and ovarian cancer, based on the probability that the individual carries a mutation on one of the BRCA genes (Berry et al 1997). The model uses the observed incidences of breast and ovarian cancer among BRCA gene mutation carriers and non-carriers to calculate the probability that a given individual is a mutation carrier based on his/her family history.
The Tyrer–Cuzick model
The Tyrer–Cuzick model identifies the risk of breast cancer in unaffected women by taking into account their probability of carrying genetic risk factors, namely a BRCA1/BRCA2 mutation; a notional common, low-penetrance dominant susceptibility allele that stands for all other genetic risk factors; and a number of other individual factors known to influence risk, such as age at menopause and menarche, weight, height, age, use of HRT, previous benign breast biopsies and parity (Tyrer et al 2004).
Future developments
The Breast Cancer Prevention Collaborative Group has advised that the following risk factors should be further examined by multivariate analysis in future studies: mammographic density, plasma hormone levels, bone density and fracture history, history of weight gain, BMI and hip–waist ratio (Santen et al 2007). It is thought that the addition of these risk factors will lead to a more accurate quantitative risk assessment model for use in future breast cancer prevention trials. Whilst breast cancer risk assessment using mathematical models based on epidemiological data is valid, no one model integrates benign breast disease, oestrogen exposure and family history in a comprehensive fashion. Therefore, it is important to use a variety of models in a specialized risk assessment clinic.
Screening, Genetic Testing and Risk Reduction
Breast cancer family history clinics are well established in the UK, and are run by medical oncologists, clinical geneticists, nurse specialists and breast surgeons in a multidisciplinary approach with close involvement of radiologists and psychologists (Evans et al 1994, 1996). After a risk assessment, women are divided into three risk groups: average, moderate and high risk. Patients should undergo pretest counselling to ensure understanding of the implications of a positive test. This should also include the risks and benefits of early cancer detection and the prevention modalities available. Counselling following testing should be available to help patients to cope with their test results and review prevention modalities. Once advised of their risk, women deciding to pursue risk reduction therapy can be presented with their management options.
Surgical management
Prophylactic/risk-reducing mastectomy (RRM) is an option for breast cancer risk reduction in high-risk women. The significant psychological and physical burden associated with RRM is reserved for those women whose lifetime risk of developing breast cancer is high. High-risk women are classified as those with a lifetime risk above 25%. This risk is the equivalent of having one first-degree relative with breast cancer diagnosed below the age of 50 years, or three affected relatives (first-degree) diagnosed under the age of 60 years. The efficacy of RRM is controversial and depends on the amount of residual tissue following the procedure. A retrospective study of 639 women with a family history of breast cancer suggests that RRM is associated with a 90% reduction in risk (Hartmann et al 1999). One small prospective study investigating the efficacy of prophylactic mastectomy in BRCA1/2 carriers with a mean follow-up of 3 years showed that those women undergoing mastectomy had a significant reduction in the incidence of breast cancer (0 of 76 women) compared with the surveillance group (eight of 63 women) (Meijers-Heijboer et al 2001).
The use of RRM is increasing, with women often choosing to have breast reconstruction surgery either immediately or delayed. The timing of the reconstruction is controversial, with immediate reconstruction appropriate in the majority of women. It is now felt that the psychological benefit of emerging from a mastectomy with a breast mound far outweighs the need for a waiting period (Kronowitz 2007). The process from first consultation to surgery takes 6–12 months; this delay deliberately allows women enough time for the decision-making process.
Bilateral prophylactic oophorectomy
Studies have shown a significant reduction in the incidence of breast cancer in women with BRCA1/2 mutations that have undergone prophylactic bilateral oophorectomy (Rebbeck et al 2002). Before undergoing the procedure, the patient should take into account how long she wishes to maintain her fertility, and should receive counselling about the risks and benefits of prophylactic oophorectomy. Opinion is divided on the use of HRT following prophylactic oophorectomy, with some centres routinely recommending HRT for all patients up to 50 years of age; the decision to use oestrogens should be based on a consideration of symptoms affecting future health and quality of life.
Chemoprevention
Chemoprevention provides a non-invasive option for breast cancer risk reduction for many high-risk women. Tamoxifen, a selective oestrogen receptor modulator (SERM) well known for its antioestrogenic effects in breast tissue, was the first agent used in breast cancer risk reduction after it was found to reduce the incidence of all breast cancers by 38% and ER-positive tumours by 48% (Cuzick et al 2003).
Pathology
In-situ carcinoma
Ductal carcinoma in situ
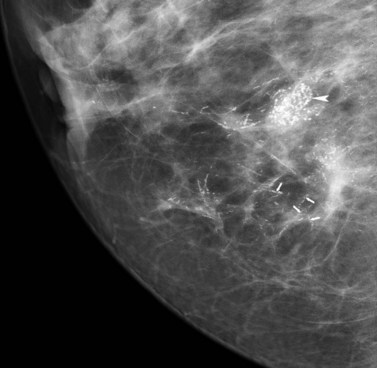
Breast screening has resulted in a marked increase in the detection of ductal carcinoma in situ (DCIS), accounting for 25–30% of all screen-detected tumours (Schwartz et al 2000). Over 90% of DCIS is impalpable, asymptomatic and often detected by screening as microcalcifications (Figure 47.1). The remaining 10% are symptomatic, presenting with nipple discharge, a palpable mass or Paget’s disease of the breast. When diagnosed clinically, DCIS is often extensive or associated with a concurrent invasive tumour. Postmortem studies have shown prevalence of DCIS from 0.2% to 14% (Bartow et al 1987, Nielsen et al 1987). Risk factors for DCIS include older age at first childbirth, nulliparity and a family history of breast cancer (Raktovich 2000). Retrospective studies of cases of low-grade DCIS misdiagnosed as benign showed that 20 years after local excision, approximately 33% had developed invasive disease (Page et al 1995). DCIS is classified into two major subtypes according to the presence/absence of comedo necrosis (atypical cells with abundant luminal necrosis that fill at least one duct) (Silverstein et al 1996). A system of low, intermediate and high nuclear grade is used to classify DCIS.
Most cases of DCIS are unicentric, with only 1% showing multicentric disease (Holland et al 1990b).
Multicentric tumour is defined as separate foci of tumour found in more than one breast quadrant or more than 5 cm from the primary tumour. Multifocal tumour is defined as more than one tumour foci in the same quadrant (Anonymous 1998c).
The spread of DCIS locally is along the branching ducts that form the glandular breast, and explains why most DCIS recurrences occur at or near the site of the initial tumour (Holland et al 1998). Micro-invasion is uncommon in DCIS, but confers a 2% risk of lymph node metastasis (van Dongen et al 1989). Studies have shown that poorly differentiated, high-grade comedo DCIS has low ER expression, high rates of cell proliferation, and overexpression of c-erbB2 (HER-2/neu) and epidermal growth factor receptor (EGFR) (Millis et al 1996). Low-grade lesions have high ER expression, lower rates of cell proliferation and rarely express HER-2 (Millis et al 1996, Boland et al 2002). Small, localized areas of DCIS (<4 cm) should be treated with breast-conserving surgery (BCS) with or without radiotherapy, and larger lesions need to be treated with mastectomy (Schwartz et al 2000).
The incidence of macroscopic nodal involvement in DCIS is less than 1%; however, nodal micrometastases have been reported in 5–14% of patients (Schuh et al 1986, Kitchen et al 2000). Two small, single-institution studies have reported a 3.1% rate of sentinel lymph node involvement in 223 patients with pure DCIS. Sentinel lymph node biopsy (SNB) for DCIS is not currently indicated but is under investigation, and may have a role in mastectomy for DCIS (Intra et al 2003). SNB should only be considered in those patients with a higher likelihood of underlying, undetected invasive disease (i.e. younger patients, DCIS >4 cm, high-grade DCIS and patients undergoing mastectomy). Twenty-five percent of cases recur within 8 years of BCS alone, 50% of which will present with invasive disease (Fisher et al 1999, Julien et al 2000, Ottesen et al 2000, Chan et al 2001); the recurrence rate for DCIS following mastectomy is 1% (Silverstein et al 1995). The key factor for recurrence is a clear margin at the time of surgery, and poor prognostic indicators include younger age at diagnosis (<40 years), poorly differentiated/high-grade tumours, presence of comedo necrosis, ER negativity and HER-2 positivity (Yen et al 2005).
The NSABP-B17, EORTC 10853 and UK/ANZ DCIS trials evaluated the value of radiotherapy following BCS for DCIS (Fisher et al 1999, Julien et al 2000, Houghton et al 2003). All of the trials reported a significant reduction in ipsilateral recurrence following radiotherapy. The EORTC trial showed a reduction in recurrence of DCIS from 8% to 5%, and a reduction in invasive recurrence from 8% to 4% at 5 years.
Lobular in-situ neoplasia
Lobular in-situ neoplasia (LISN; formerly known as lobular carcinoma in situ) is not itself a premalignant lesion but is a high-risk marker of invasive cancer. It is often an incidental finding during breast biopsy, and accounts for 0.5% of symptomatic and 1% of screen-detected tumours. Patients with LISN are younger, premenopausal with bilateral and multicentric disease of lower grade. Almost all patients express ER (Akashi-Tanaka et al 2000). If LISN is detected at core biopsy, the area should be subjected to excision biopsy to confirm the diagnosis and exclude an invasive focus. The NSABP P-1 prevention trial reported a 56% risk reduction of developing subsequent invasive cancer with tamoxifen in patients with a history of LISN (Dunn and Ford 2000).
Invasive carcinoma
Invasive lobular carcinoma
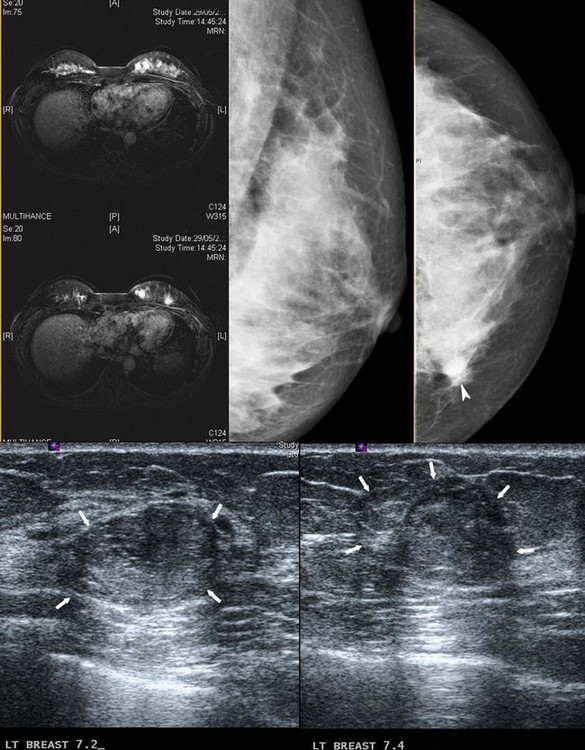
Invasive lobular carcinoma (ILC) is the second most common type of invasive breast cancer accounting for 8–14% of all invasive breast cancers (Arpino et al 2004). ILC clinically presents as a poorly defined thickening of the breast rather than a dominant mass. This makes the extent of the disease difficult to estimate on clinical examination, and difficult to visualize on mammography. Ultrasonography is more sensitive than mammography in detecting ILC, but may significantly underestimate the size of the lesions (Pritt et al 2004, Selinko et al 2004). Magnetic resonance imaging (MRI) is more accurate than either mammography or ultrasonography in defining the extent of the disease but is less widely available (Weinstein et al 2001). Due to the infiltrative growth pattern, there is a higher incidence of resection margin involvement than for IDC and a higher conversion rate to mastectomy (Yeatman et al 1995). ILC has a higher incidence of being bilateral, multifocal and multicentric than IDC (Figure 47.2) (Ashikari et al 1973). Axillary lymph nodes in ILC may remain impalpable even when extensively involved (Grube et al 2002). A large study from the National Cancer Database to examine treatment and outcomes in patients with ILC showed a similar 5-year survival rate between patients with ILC and IDC, and between those ILC patients receiving breast-conserving therapy and those receiving mastectomy (Winchester et al 1998).
Paget’s disease of the breast
Sir James Paget (1874) described ‘an eczematous change in the skin of the nipple preceding an underlying mammary cancer’, now known as Paget’s disease (Paget 1874). More than 95% of women with Paget’s disease of the nipple have an underlying malignancy, although 50% are clinically and mammographically undetectable (Vielh et al 1993). Paget’s disease accounts for 0.7–4.9% of all breast malignancies, and misdiagnosis as eczema and treatment with topical steroids explains an average 10–12-month delay in diagnosis (Kister and Haagensen 1970, Lagios et al 1984, Chaudary et al 1986, Dixon et al 1991, Kothari et al 2002). The first symptoms include burning, itching and change in sensation of the nipple–areola complex; raised skin lesions follow this, with a clear demarcation from the surrounding skin. Rarely, nipple deformity and retraction may occur if there is tethering from an underlying malignancy. Characteristically, it starts on the nipple and spreads to the areola and then surrounding skin, and in the later stages may present with ulceration and bleeding. Differential diagnoses include chronic eczema, benign papilloma of the nipple, basal cell carcinoma, Bowen’s disease and malignant melanoma (Jamali et al 1996).
A full-thickness punch biopsy of the nipple should be performed to confirm the diagnosis (Rosen 1996). Mammography, ultrasonography and MRI can be used to detect an underlying mass, as well as assessing the contralateral breast. Paget’s disease often presents with multicentric disease (75%); therefore, mastectomy is advocated as the procedure of choice (Kothari et al 2002). However, several small studies of BCS and radiotherapy for Paget’s disease have shown low rates of recurrence (Fourquet et al 1987, Dixon et al 1991, Kollmorgen et al 1998, Bijker et al 2001, Fu et al 2001, Marshall et al 2003). Attempts to preserve uninvolved areas of the nipple are associated with high rates of local recurrence (Stockdale et al 1989, Fu et al 2001). Staging by SNB or axillary dissection is required for all patients with invasive disease.
Inflammatory breast cancer
Inflammatory breast cancer (IBC) was first coined by Lee and Tannenbaum at the Memorial Hospital, New York in 1924 to describe an aggressive form of breast cancer with an incidence of 1–6% (Lee and Tannenbaum 1924, Levine et al 1985). A higher incidence is reported in African-Americans than in Caucasians and other ethnic groups (10.1%, 6.2% and 5.1%, respectively). Women with IBC often present at a younger age than women with non-IBC (NIBC) (Chang et al 1998, Anderson et al 2003, Charafe-Jauffret et al 2004). The 10-year survival rate of patients with IBC is 26.7%, compared with 44.8% for those with NIBC (Low et al 2002). A recent review of 635 patients at the M.D. Anderson, Texas (IBC, n = 214; stage III NIBC, n = 421) demonstrated a significantly reduced progression-free survival for IBC compared with NIBC (24 months and 35 months, respectively), and of overall survival for IBC compared with NIBC (42 months and 60 months, respectively).
The criteria for diagnosis was described by Haagensen including erythema, oedema involving more than two-thirds of the breast, tenderness, induration, warmth, enlargement, peau d’orange and diffuseness of the tumour on palpation (Haagensen 1971). These symptoms progress rapidly. At diagnosis, most patients (55–85%) have axillary lymph node involvement and up to 30% have distant metastases (Jaiyesimi et al 1992, Hance et al 2005). Pathology shows extensive lymphovascular invasion by tumour emboli, involving the superficial dermal plexus of vessels in the papillary and high reticular dermis (Taylor and Meltzer 1938). Primary IBC is the development of carcinoma and skin changes in a previously healthy breast, and secondary IBC is the development of inflammatory IBC in a breast that has had a previous malignancy or changes caused by irradiation.
One treatment protocol from the M.D. Anderson advises initial treatment with neoadjuvant sequential taxane (paclitaxel/docetaxel) and an anthracycline-based regimen (5-fluorouracil + epirubicin + cyclophosphamide) (Cristofanilli et al 2002). Patients who show a partial or complete response undergo surgery (mastectomy and axillary dissection) followed by adjuvant radiotherapy and hormonal therapy (if ERα-positive). For those patients who do not respond, radiotherapy to the breast and axilla is recommended, followed by surgery, if feasible, and adjuvant hormonal therapy (if ERα-positive) (Singletary 2008). SNB is not recommended in patients with IBC as the identification rate is only 70% and the false-negative rate is 40% (Stearns et al 2002). Currently, most practitioners recommend a delayed reconstruction after completion of therapy. Most local recurrences occur in the mastectomy flap.
A number of studies have reported a higher incidence of c-erbB2 (HER-2) in patients with IBC (Turpin et al 2002, Parton et al 2004). One small study (n = 22, IBC = 9, NIBC = 13) combining docetaxel with trastuzumab as part of the primary systemic therapy observed a complete response in 40% of patients (van Pelt et al 2003). Lapatinib (reversible inhibitor of c-erbB1 and c-erbB2) has shown partial responses in women with IBC who have been extensively pretreated (Burris et al 2005, Spector et al 2006). The combination of lapatinib with paclitaxel in a cohort of 21 chemotherapy-naïve IBC patients overexpressing HER-2 showed a clinical response rate of 95% (Cristofanilli et al 2006).
Other breast malignancies
Primary cutaneous melanoma of the skin of the breast is very rare, accounting for 0.28% of all cases of melanoma (Ariel and Caron 1972). It is more commonly found as a result of a distant metastasis from a primary elsewhere. A full history and physical examination for the presence of other melanomas should be performed. A core or surgical biopsy of the thickest portion of the melanoma will determine the depth of invasion. Immunostaining for HMB-45 and S100 enables differentiation of melanoma from other cutaneous masses. Staging is with computed tomography (CT) or positron emission tomography (PET), and lactate dehydrogenase levels should be measured. Wide local excision is recommended with margins dependent upon the depth of invasion, and SNB performed at the time of excision. Complete axillary dissection should be performed for those patients with clinically suspicious lymphadenopathy and those with melanomas larger than 4 mm (Essner et al 2002). Adjuvant therapy is similar to that for melanoma elsewhere in the body.
Radiation-induced primary angiosarcoma of the breast, first reported in 1981, is extremely rare (Maddox and Evans 1981). Approximately 60 cases have been reported, although the incidence is rising (Rao et al 2003). It presents as violet, blue, red or black skin nodules in a multifocal pattern, and is diagnosed by a punch, incisional or excisional biopsy (Fineberg and Rosen 1994). Fine needle aspiration, mammography and ultrasonography are often not helpful. Factor VIII-related antigen is positive in most angiosarcomas, distinguishing it from other sarcomas (Stokkel and Peterse 1992). Staging is based on the size, grade and depth of the tumour, with grade being the most important prognostic indicator. Most angiosarcomas are high grade and are treated with mastectomy (Fineberg and Rosen 1994). Axillary staging is not required, as most sarcomas do not metastasize to regional lymph nodes. The recurrence rate is 40% and prognosis is poor.
Primary breast lymphoma (PBL) is defined as a lymphoma localized to the breast and its draining basins, arising from lymphoid tissue in the breast (Smith et al 1987, Kuper-Hommel et al 2003). It accounts for 0.14% of all breast malignancies and 0.65% of all non-Hodgkin’s lymphomas, with the most common type being diffuse large B-cell lymphoma (Ha et al 1998, Kuper-Hommel et al 2003). PBL arises most commonly in the seventh decade as a painless, enlarged rubbery mass. Mammographic and ultrasound findings are non-specific, and PBL is categorized according to the Ann Arbor classification of lymphomas. Diagnosis is by core biopsy, and the patient is staged by CT of the neck, chest, abdomen and pelvis, and bone marrow biopsy. Surgery with adjuvant radiotherapy is no longer advocated due to the high incidence of local and disseminated recurrence (Kuper-Hommel et al 2003). Treatment of PBL is now with chemotherapy, followed by targeted radiotherapy, with a 2-year survival rate of 63% (Ha et al 1998, Brogi and Harris 1999).
Metastasis to the breast
This is uncommon and accounts for 0.5–6.6% of breast malignancies, with the contralateral breast being the most common site of metastatic malignancy (Bohman et al 1982, Paulus and Libshitz 1982, Amichetti et al 1990). Other malignancies that metastasize to the breast include lymphoma, melanoma, rhabdomyosarcoma and small cell lung cancer (Bartella et al 2003
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree