Malignant Changes of the Female Genital Tract
4
Malignant Tumors of the Vagina
Tumors Spreading from Adjacent Organs
Metastatic Tumors of the Genital Tract
| 4 | Malignant Changes of the Female Genital Tract |
Portio and Vagina
Epidemiology
Thanks to the effectiveness of cancer screening programs, invasive cervical carcinoma is rare today (0.1–0.2%o). Severe precancerous stages, however, occur roughly 10 times as frequently, and less severe stages as much as 100 times as frequently.
Incidence. Currently, at the start of the 21st century, the relative frequency of cervical carcinoma worldwide is 0.1-0.2%o. This means that one or two out of 10 000 women examined each year are affected by this disease (355). In the late 1960s, this figure was still 0.3–0.4%o, more than twice as high as it is today (Fig. 4.1) (7a, 118a).
Cytological examinations aimed at the early detection of cervical cancer have been carried out since the early 1950s (51), and prescriptive screening has been available in Germany since 7 July 1971. This means that women aged 20 and above are entitled to have such an examination once a year at the expense of the national health insurance scheme (166).
Prevalence. The absolute frequency of cervical carcinoma is higher than the incidence because it includes all women, not just those who have undergone cytological testing. Currently, the prevalence is 0.3–0.4%o worldwide (81).
Early detection. The population participation rate for prescriptive screening for early detection of cancer varies from country to country; in Germany it is 50–60% (316, 366). It should be taken into account, however, that women who undergo cytological testing include not only those who participate in prescriptive screening but almost all those who have a gynecological examination for other reasons such as contraception, pregnancy, or menopausal symptoms. It is therefore difficult to estimate the actual percentage of women who undergo cytological testing more or less regularly, and this percentage is probably much higher than that for early detection screening. Nevertheless, failure to participate in regular screening for cancer prevention is the highest risk factor possible for the development of cervical carcinoma (62). The risk is six times higher in this group than for women who are regularly monitored.
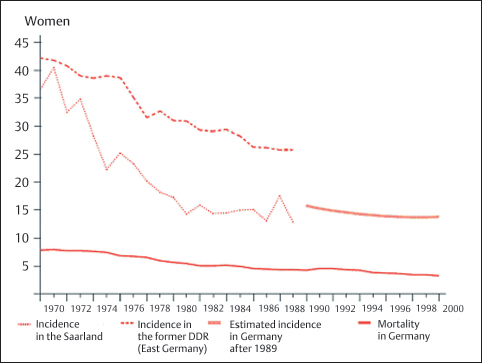
Fig. 4.1 Incidence and mortality of cervical carcinoma in Germany between 1970 and 2000. Age-adjusted incidence and mortality per 100 000 women screened each year. (Robert Koch Institute, Berlin, Germany, see ref. 7a.)
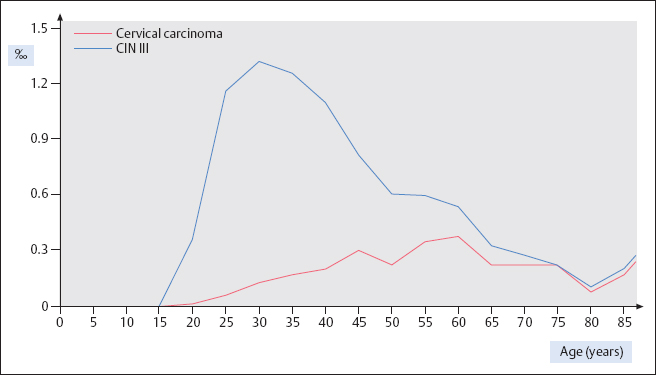
Age distribution. The age distribution of women affected by cervical carcinoma shows a peak at age 60, and the frequency is about the same at ages 40 and 70 (355). However, grade III cervical intraepithelial neoplasia (CIN III) occurs four times more often before the age of 40 than after age 70 (Fig. 4.2).
Precancerous stages. The incidence of precursors of cancer differs significantly from that of invasive cervical carcinoma, being about 100 times higher (312). The incidence of mild to moderate dysplasia is about 1 %, and that of severe dysplasia and carcinoma in situ is about 2%o (Fig. 4.3). The peak of precancerous stages occurs at age 30, which is 30 years earlier than for invasive carcinoma (327). There is a steep increase starting at age 15 and a slow, steady decline after age 30 and into old age (Fig. 4.2).
Factors influencing epidemiology. Over the last 50 years, the epidemiology of cervical carcinoma—and that of its precursors, in particular—has been clearly influenced by two factors: the successful screening for early detection, on the one hand, and the increasing endemic infection of the population with human papillomavirus (HPV), on the other. Timely detection of severe precancerous stages leads to their therapeutic elimination before a carcinoma develops (355). The increased frequency of HPV infection, however, has caused a dramatic increase in mild and moderate dysplasia, and these stages are usually not removed right away but only monitored at regular intervals. These circumstances have resulted in a distinct shift of incidence rates within the last decades.
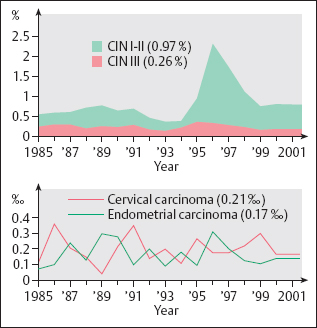
Fig. 4.3 Frequency distribution of positive Pap smears, based on 1.14 million cytological smears evaluated between 1985 and 2001 (Cytological Laboratory of Prof. Nauth, Stuttgart, Germany). The peak of the CIN I–II findings during 1996/1997 is attributed to a change in the method of smear collection at this time (using a Szalay spatula instead of a cotton swab), which caused a temporary increase in positive findings (particularly of CIN I–II lesions) due to overrating.
Morphogenesis
The current view is that cervical carcinoma is caused by HPV infection. Carcinogenesis takes place via several intermediate stages known as dysplasia or cervical intraepithelial neoplasia (CIN). The last of these stages (CIN III) is difficult to distinguish from carcinoma in situ (CIS).
In terms of chronological development, we distinguish:
- Mild dysplasia (CIN I)
- Moderate dysplasia (CIN II)
- Severe dysplasia (CIN III)
- Carcinoma in situ (CIN III/CIS)
- Invasive carcinoma
In terms of morphological development, we
distinguish:
- Nonkeratinizing dysplasia
- Metaplastic dysplasia
- Keratinizing dysplasia
- Small-cell dysplasia
- Glandular dysplasia (see pp. 214ff.)
Several forms of dysplasia often exist simultaneously, side by side. The morphogenesis of squamous cell carcinoma and that of endocervical adenocarcinoma cannot be evaluated in isolation from each other.
Invasive squamous cell carcinoma of the cervix shows the following growth patterns, which may exist simultaneously:
- Large-cell squamous carcinoma
- Keratinizing squamous carcinoma
- Small-cell squamous carcinoma
HPV Infection
There are numerous hypotheses for the development of cervical carcinoma, and they involve factors ranging from promiscuity, parity, genital hygiene, nutritional habits, and addiction—particularly cigarette smoking—to viral infections (208).
Transmission. Current discussions focus on HPV infection, which is the most likely candidate for being involved in cervical carcinogenesis (147, 381). Certain HPV subtypes, particularly types 16 and 18, are thought to be the real cause of cervical cancer (82). Since this virus is transmitted by sexual contact, specific risk factors include an early start of sexual activity, a high number of sexual partners, and low socio-economic status (142).
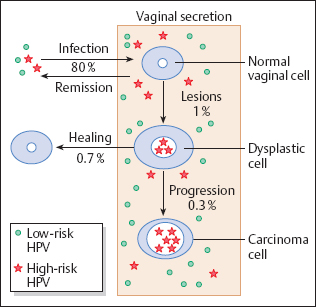
It is now assumed that 80 % of the population come into contact with HPV for shorter or longer periods of time during their lives (382), and that about 10% of all women become infected with high-risk types of HPV (see also p. 102). However, only 1 % of this group develop lesions, and about 70 % of these undergo spontaneous healing (Fig. 4.4).
Route of infection. The virus turns virulent when penetrating the epithelial barrier, thus being able to infect cells that are still capable of dividing, namely, basal cells, immature metaplastic cells, and reserve cells (81). The virus is most effective when the epithelium has already been damaged by bacterial or fungal infections, as this facilitates the penetration of virus particles into the epidermis (221). This pathway of infection is particularly facilitated on the boundary between squamous epithelium and columnar epithelium of the portio vaginalis where the metaplastic process of transformation predominates (342).
Viral replication. The shell of protein that protects the viral genome, the capsid, disintegrates upon entrance of the virus into the host cell, and the naked viral DNAbegins to replicate (productive infection). Because of the cell’s greater metabolic needs, the nucleus increases in size and becomes polyploid. Cytologically, this change correlates with mild to moderate dysplasia. During the terminal phase of squamous epithelial differentiation, the capsid proteins, or late proteins (L1 and L2) are synthesized, thus ensuring survival and infectiousness of the virus after exfoliation and disintegration of infected superficial cells (8, 221a). Occasionally, however, the viral DNA becomes integrated into the genome of the host cell; this amounts to a genetic “accident” as the virus is now unable to replicate (nonproductive infection). Now, only some early proteins are synthesized, but late proteins are suppressed, thus evading the host’s immune response. The early proteins E6 and E7, specifically, act as oncoproteins by inactivating tumor suppressor proteins and stimulating cell proliferation (429). Cytologically, these changes correlate with severe dysplasia or carcinoma in situ. At this stage of carcinogenesis, certain components of tobacco smoke—when excreted at high concentration into the cervical mucus—possibly act as cocarcinogens by further raising the rate of cell division (252).
Controlled carcinogenesis. For variable periods of time, the host cell’s regulatory mechanism is able to recognize genomic damage caused by mutation. The damaged cells are then eliminated by means of DNA repair and subsequent controlled cell death, or apoptosis, mediated by the tumor suppressor genes p16 and p53. This process is called controlled carcinogenesis(Fig. 4.5) (73a, 173a and b, 350).
Uncontrolled carcinogenesis. Over longer periods of time, the mutagenic effects of the virus may disturb certain short nucleotide sequences, so-called microsatellites, in the host cell’s DNA repair genes. The resulting microsatellite instability interferes more and more with the normal function of these repair genes. A high degree of instability finally leads to uncontrolled cell proliferation, thus promoting the induction of secondary mutations that are no longer eliminated through DNA repair. Thus, the development of cancer can no longer be prevented, even if the virus were to be eliminated at this stage. This process is called uncontrolled carcinogenesis(Fig. 4.6) (350).
Koilocytes. The virus-infected basal cells mature into superficial cells during their migration through the epithelium. At the intermediate and superficial cell stages, viral metabolites are released from the nuclei and cause damage in the cytoplasm, thus creating irregular perinuclear halos. Cells affected in this way are called koilocytes (15, 181) (see also p. 102).
Dyskeratocytes. The basal cells respond to HPV infection with enhanced proliferation. This results in accelerated epithelial maturation and may be accompanied by temporary keratinization on the surface. Koilocytic intermediate and superficial cells mature into atypical horn cells, called dyskeratocytes. These finally exfoliate and release HPV particles from their nuclei when they undergo cytolysis (see also p. 102). Reinfection, or the infection of a sexual partner, is therefore possible (220).
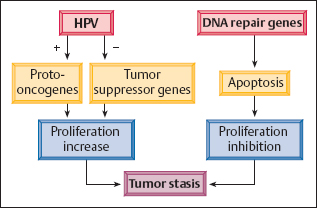
Fig. 4.5 Controlled carcinogenesis. Long-term dysplasia is associated with tumor stasis, a state in which the increase in HPV-stimulated cell proliferation is inhibited by the host cell’s defense mechanism, through the activation of DNA repair genes.
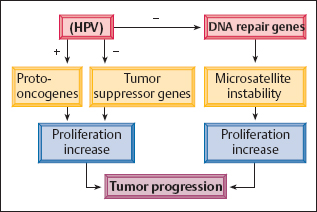
Fig. 4.6 Uncontrolled carcinogenesis. Tumor progression occurs when HPV damages the body’s repair genes, thus preventing the inhibition of increased cell proliferation.
Contributing factors. The aggressiveness of HPV depends on the subtype, but also on certain factors that are not yet well understood. The body’s immune status probably plays a major role (184). This explains why cervical neoplasms occur more frequently in connection with diseases that weaken the immune system and, in particular, with HPV infection (85a). As maturation of the squamous epithelium usually ceases after the menopause, as a result of hormonal deficiency, the virus is no longer able to attack during this period of life. Hence, the frequency of cervical carcinoma declines with age (355).
Types of HPV. Infection with certain subtypes of the virus, particularly types 6 and 11, causes pronounced thickening of the superficial zone of keratinization, thus creating warts, or condylomas. These are epithelial proliferations without precancerous potential (218, 381) (see also pp. 162 ff.). Other types of HPV, mainly types 16 and 18, stimulate the basal cells so intensely that they respond not only with increased proliferation but also with nuclear abnormalities.
Dysplasia
Development of dysplasia. The layer of abnormal basal cells thickens over the years, and the dysplastic basal cells have less and less time for maturation (Fig. 4.7) (187). They are therefore still at a relatively low level of differentiation when they reach the epithelial surface. Over the years, exfoliated cells are more and more immature, and koilocytic features become rare as they only occur in mature cells. The condition associated with the increased thickness of the abnormal basal cell layer and the resulting disturbed maturation in the upper cell layers of the squamous epithelium is called dysplasia(313).
Grades of dysplasia. Depending on the severity of the precancerous stage, the condition is classified as mild, moderate, or severe dysplasia (CIN I, II, orIII) (311). The Germannomenclature distinguishes an additional stage, carcinoma in situ (preinvasive carcinoma) (48).
Types of dysplasia. The morphology of dysplasia differs depending on which type of cells—basal cells, immature metaplastic cells, or reserve cells—has been infected (246a, 281). The further differentiation of these cell terms in different types of carcinomas may also play a role (Fig. 4.8).
In about 70% of patients with dysplasia, basal cells or immature metaplastic cells are infected, and this results in nonkeratinizing or metaplastic dysplasia(Fig. 4.9).
In about 20% of cases, the dysplastic stage is skipped due to infection. This possibly happens when preexisting inflammation causes basal cell hyperplasia and the concurrent HPV infection prevents the epithelium from maturing. The stage of abnormal basal cell hyperplasia thus leads directly to the development of small-cell carcinoma in situ(Fig. 4.10).
In another 7%, the infection causes superficial keratinization, or dyskeratosis, of the abnormal epithelium. This is called keratinizing dysplasia (328), and its structure resembles vulvar dysplasia (see Fig. 4.105, p. 241).
In the remaining 3%, the reserve cells of the columnar epithelium are infected. The resulting abnormal reserve cell hyperplasia then leads to glandular dysplasia and, finally, to endocervical adenocarcinoma in situ (Fig. 4.11) (403) (see pp. 214 ff.). However, the actual frequency of adenocarcinoma and its precursors seems to be higher (about 10%) than can be detected by cytological means (248a). Because glandular cells tend to exfoliate very little, and their criteria for malignancy are difficult to detect, glandular dysplasia is probably not always recognized cytologically.
The various morphological types ofdysplasia may develop independently of one another, and their morphogenesis is often mixed (104).
Regression of dysplasia. In principle, dysplastic lesions are reversible, and regression probably depends on the body’s immune status. About 60–70% of all mild dysplasia will regress within a year and turn into normal epithelium (239). For higher grades of dysplasia, however, regression is less likely (371). Nevertheless, the frequency of regression for CIN III lesions is assumed to be up to 30 % (267a).
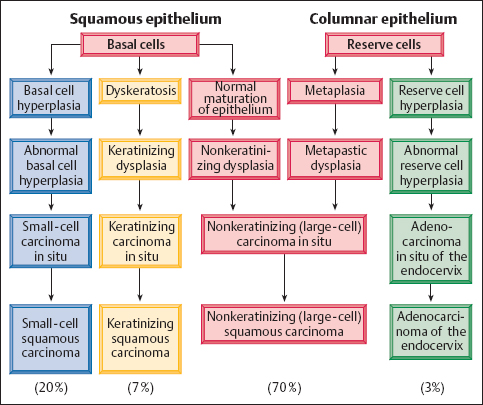
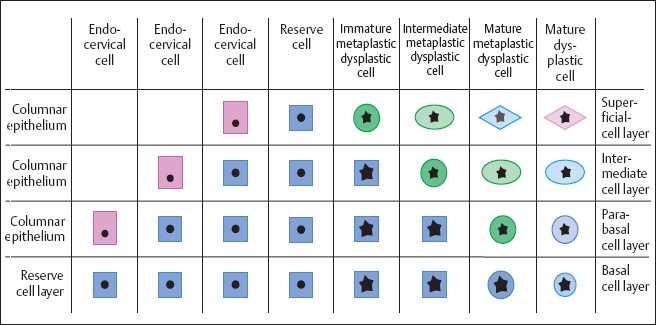
Fig. 4.9 Development of metaplastic dysplasia. When HPV lesions occur in the endocervical epithelium during metaplastic transformation, abnormal reserve cells develop first, followed by abnormal metaplastic cells of various degrees of maturation. This finally leads to fully developed dyskaryosis of the squamous epithelium.
Duration of dysplasia. The precancerous stages may last for various lengths of time. If dysplasia gives rise to a carcinoma, this will be preceded by mild dysplasia for 6 years on average, moderate dysplasia for 4 years, and severe dysplasia for about 1 year (312, 367). If the epithelium already consists of nothing but immature abnormal cells, the lesion has a homogeneous, monotonous structure. This state, called carcinoma in situ, then exists for another period of 5–15 years on average (175, 362). There are, however, indications that these periods show considerable variation (18a). As a rule, it will take at least 6 months for mild dysplasia to develop into severe dysplasia (413a). The small-cell variant of CIN III lesions seems to have a very brief course, since it does not have a dysplastic precursor stage but develops fairly suddenly from an inflammatory state of basal cell hyperplasia. Its frequency of about 20% correlates with the practical experience with cases of CIN III: previous cytological findings have been positive (CIN I, II) in only about 50% of cases and negative in 25 %, whereas there were no findings in another 25 %. Hence, it can be assumed that at least one quarter of all CIN III lesions develop much faster than previously thought.
Tumor invasion. At some point, the abnormal epithelium no longer expands further toward the surface but begins to produce enzymes known as proteases. These enzymes dissolve the basal membrane, which has so far represented the last barrier between epithelium and connective tissue. This enables the abnormal cells to infiltrate the stroma; this process is called tumor invasion (285, 407). By definition, the microinvasive carcinoma thus formed has a depth of invasion of 3 mm or less and a horizontal spread of 7 mm or less (56, 204). By contrast, a microcarcinoma is defined as having an invasive depth of 5 mm or less and a horizontal spread of 7 mm or less (169).
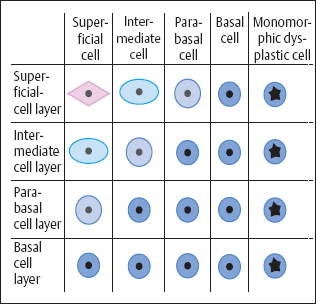
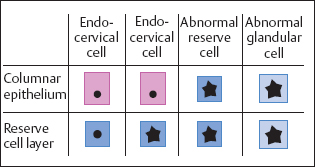
Fig. 4.11 Development of adenocarcinoma in situ. A HPV lesion in the endocervical columnar epithelium initially leads to abnormal reserve cells and then to abnormal glandular cells.
Once there is a clinically manifest carcinoma, the tumor cells aggressively attack the lymphatic vessels and blood vessels by releasing enzymes known as collagenases. This facilitates their metastatic spread to the lymph nodes and organs (11). Here, they produce vasoendothelial growth factors, which provide for their own vascular supply (2). Because of their enormous growth potential, the metastases occupy more and more space at the expense of healthy tissues, thus leading to the death of the patient.
Vaginal carcinoma. Morphogenesis of vaginal carcinoma probably takes place in a similar way as in cervical carcinoma, with the same histological and cytological changes occurring also in the vaginal epithelium, though far less often (see p. 212).
Colposcopy
The precancerous and early stages of cervical carcinoma show characteristic colposcopic changes, especially when the acetic acid test is applied:
- Acetowhite epithelium
- Punctuation
- Mosaic pattern
- Abnormal transformation zone
- Prominent leukoplakia
- Abnormal vascularization
- Tumor or ulcer
The clinical symptoms of epithelial cancer development are nonspecific, and there is usually no discomfort involved. During its precancerous stages, however, a developing cervical carcinoma exhibits characteristic manifestations under the colposcope. Here, both the acetic acid test and the iodine test are valuable diagnostic tools.
- Acetowhite epithelium: Dysplasia developing within the original squamous epithelium consists of nonkeratinizing cells and is therefore completely inapparent in the native condition. It turns temporarily white, however, upon topical application of acetic acid (see p. 29).
- Punctuation: When the epithelial dysplasia intensifies, the stromal papillae are already visible as red dots before the acetic acid test, but they become more prominent after application of acetic acid (Figs. 4.12, 4.13) (223).
- Mosaic pattern: If the dysplasia is severe, the epithelial cones become much broader as they grow toward the stroma. As a result, the laterally compressed stromal papillae appear as narrow red ridges on the surface of the epithelium, where they create a mosaic pattern (Figs. 4.12, 4.14). Here, too, the acetic acid test intensifies the pattern, whereas the iodine test is negative (405).
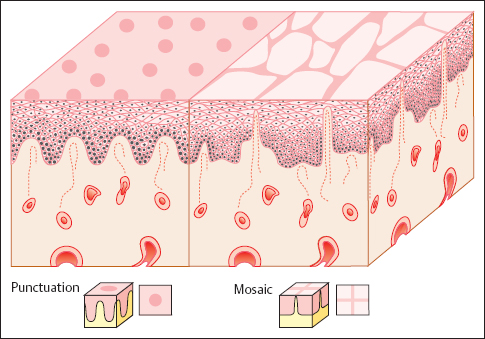
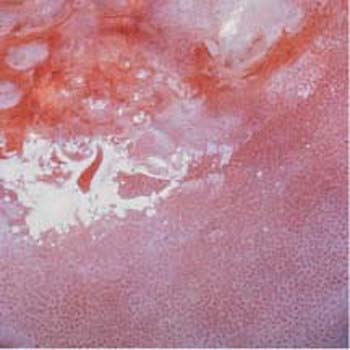
Fig. 4.13 Area with abnormal punctuation after application of the acetic acid test. The area below the transformation zone constitutes a CIN I or CIN II lesion.
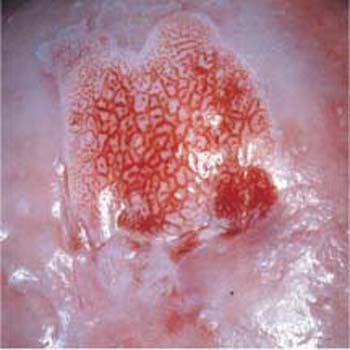
Fig. 4.14 Area with abnormal mosaic pattern after application of the acetic acid test. The area in the center of the prominent cervical leukoplakia constitutes a CIN III lesion.
- Abnormal transformation zone: If a carcinoma develops within the metaplastic epithelium of the transformation zone, the epithelial structure becomes irregular and contains areas of both erythroplakia and leukoplakia. The glandular openings appear particularly prominent and stick out like buttons. This pattern is called an abnormal transformation zone (Fig. 4.15) (404) and results in an uneven surface of the epithelium. Application of acetic acid causes distinct acetowhitening (Fig. 4.16a, b), whereas the iodine test is negative (54).
- Prominent leukoplakia: In keratinizing dysplasia, a flaky, prominent leukoplakia already exists before the application of acetic acid. The extent of the leukoplakia does not permit any conclusions regarding the severity of the dysplasia, because the keratinized layer acts as a filter and makes details deep in the epithelium invisible (Fig. 4.17) (54).
- Abnormal vascularization: Under the colposcope, invasive carcinoma is characterized by abnormal vascularization with bizarre ramification and changing caliber of the vessels (Fig. 4.18) (337, 372). Furthermore, there may be a tendency for bleeding and the tissue may be fragile (171).
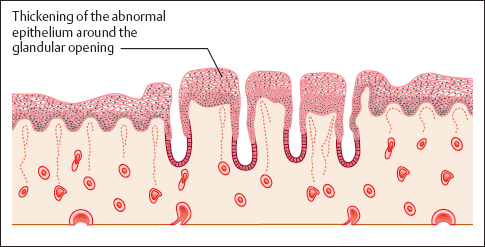
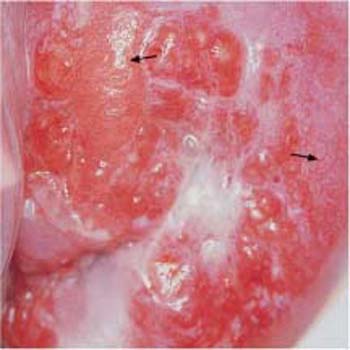
Fig. 4.16a, b Abnormal transformation zone.
a The prominent zone with irregular areas of leukoplakia constitutes a CIN III lesion. Areas with mosaic pattern or punctuation are visible as well (→)
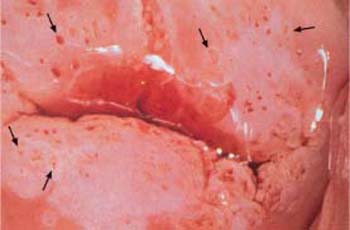
b The prominent zone with circular thickening around the glandular openings (→) constitutes a CIN III lesion.
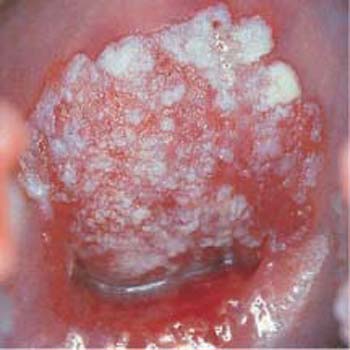
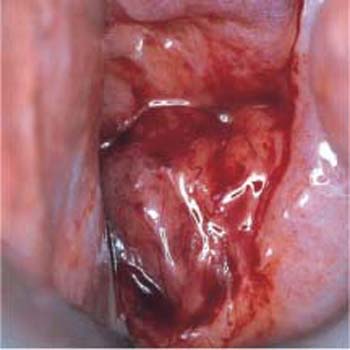
Fig. 4.18 Abnormal vascularization. The wide variation in vascular caliber and the irregular ramifications of blood vessels on the anterior and posterior lips of the cervical os are caused by an invasive cervical carcinoma.
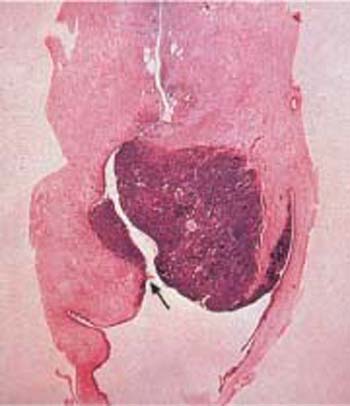
Fig. 4.19 Squamous cell carcinoma of the cervix. Histological gross section. The carcinoma grows at the level of the portio vaginalis while spreading and infiltrating deep into the tissue, thus pushing the cervical canal (→) to the left of the photograph. HE stain, actual size.
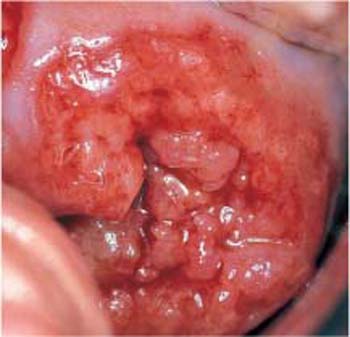
Fig. 4.20 Irregular crater-shaped ulcer due to invasive squamous cell carcinoma of the cervix.
- Tumor or ulcer: The carcinoma may form an exophythic tumor, infiltrate the stroma in the area of the portio vaginalis (Fig. 4.19), or cause a well-defined ulcer due to superficial necrosis (Fig. 4.20) (405). The acetic acid test and the iodine test show complementary reactions, thus providing diagnostic clues. Usually, it is only at this late stage of cancer development that the first clinical symptoms appear in the form of irregular vaginal bleeds due to vascular erosion.
Histology
The precursors of cervical cancer are subdivided into four stages: mild, moderate, and severe dysplasia, and carcinoma in situ (preinvasive carcinoma). The international nomenclature does not differentiate between severe dysplasia and carcinoma in situ and defines only three stages: cervical intraepithelial neoplasia (CIN) grades I–III. Independently of this subdivision by grade, a distinction is made between nonkeratinizing, meta-plastic, keratinizing, small-cell, and glandular dysplasia. The various types of invasive cervical carcinoma are defined as nonkeratinizing (large-cell), keratinizing, and small-cell carcinoma.
Grades of dysplasia. Squamous epithelial carcinogenesis affects primarily the original nonkeratinizing epithelium. The histological criteria for the degree of malignancy of the dysplasia depend on the vertical expansion of the abnormal basal cell layer. This layer is not only thicker than in the normal epithelium, but its nuclei are polymorphic, enlarged, and hyperchromatic and exhibit an abnormal chromatin structure and increased mitotic rates (22, 305). The WHO classification distinguishes between the following grades:
- Mild dysplasia (CINI): the thickening of the abnormal epithelium is restricted to the lower third of the epithelial layer (Fig. 4.21a).
- Moderate dysplasia (CIN II): the lower two thirds of the epithelial layer are abnormal (Fig. 4.21b).
- Severe dysplasia (CIN III): the upper third of the epithelial layer is abnormal as well (Fig. 4.21c) (311, 313).
Koilocytes are detected not only in CIN I and CIN II lesions but sometimes also in the upper cell layers of CIN III lesions, although these usually do not exfoliate (Fig. 4.22).
Carcinoma in situ. When there is no further maturation and the epithelium becomes monomorphic, the lesion is called carcinoma in situ. However, its biological significance does not differ from that of a severe dysplasia. Hence, it is usually referred to as CIN Ill/CIS (Fig. 4.23). Severely abnormal squamous epithelium frequently lines the cervical glands (Fig. 4.24), although these are normally lined with columnar epithelium. It was originally assumed that the abnormal squamous epithelium grows into the glands to replace the columnar epithelium. However, recent studies suggest that reserve cells underneath the columnar epithelium transform into abnormal basal cells after infection with HPV and then differentiate into squamous epithelial cells (21).
Types of dysplasia. In nonkeratinizing dysplasia the epithelium contains abnormal basal cells, whereas metaplastic dysplasia is distinguished by the presence ofabnormal metaplastic cells (Fig. 4.25) (28). In keratinizing dysplasia, the different degrees of severity are histologically distinct, but the superficial horny layer prevents exfoliation of the deeper layers of the epithelium, thus making the cytological diagnosis difficult (Fig. 4.26) (368). When a carcinoma does not develop via dysplasia but rather via abnormal basal cell hyperplasia, a small-cell carcinoma in situ develops relatively suddenly, and this is often referred to as CIN III of the basal cell type (Fig. 4.27) (22).
Types of carcinoma. The classic histological nomenclature (309) distinguishes between the following growth types of invasive cervical 4 carcinoma:
- Nonkeratinizing (large-cell) squamous carcinoma
- Keratinizing squamous carcinoma
- Small-cell squamous carcinoma (Figs. 4.28–4.30) (306)
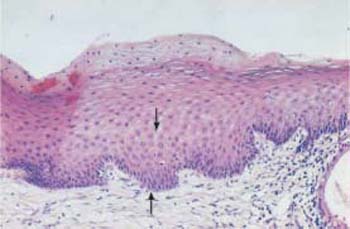
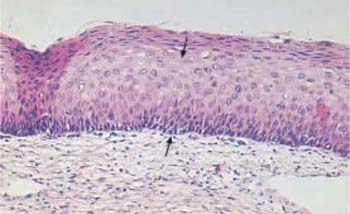
b Moderate dysplasia. The basal cell layer consists of immature cells with enlarged, polymorphic, and hyperchromatic nuclei. The thickening of the basal cell layer occupies the lower two thirds of the epithelium (→). HE stain, ×100.
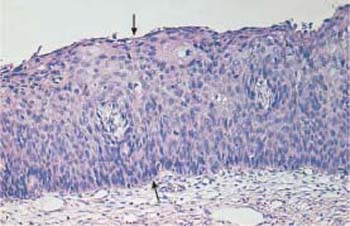
c Severe dysplasia. The basal cell layer consists of immature cells with enlarged, polymorphic, and hyperchromatic nuclei. The thickening of the basal cell layer occupies the total width of the epithelium (→). Only at the very surface is there a cell layer with condensed nuclei and signs of keratinization—a condition called pseudomaturation. HE stain, ×100.
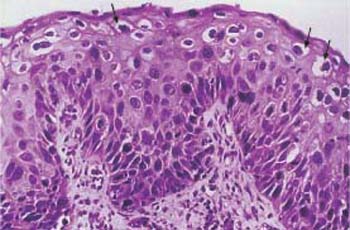
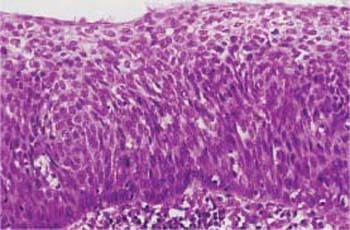
Fig. 4.23 Large-cell carcinoma in situ of the cervical epithelium. The abnormal epithelium consists of immature cells with enlarged, hyperchromatic, but monomorphic nuclei. The thickening of the basal cell layer occupies the total width of the epithelium. The uniform size of the cells gives the epithelium a monomorphic character. HE stain, ×400.
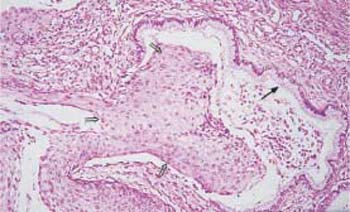
Fig. 4.24 Cervical glands lined with severely dysplastic cervical epithelium. While there is still a normal, single-layered columnar epithelium visible on the right (→), it has been replaced by abnormal squamous epithelium on the left (⇒). HE stain, ×100;.
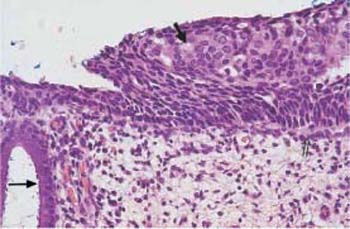
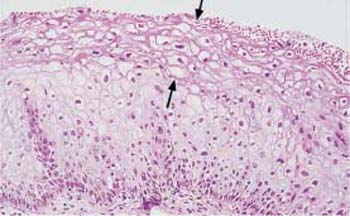
Fig. 4.26 Mild keratinizing dysplasia of the cervical epithelium. The basal cell layer consists of immature cells with enlarged, polymorphic, and hyperchromatic nuclei. The thickening of the basal cell layer remains restricted to the lowerthird of the epithelium, although individual abnormal cells do reach the surface. Superficially, there is a relatively thick dyskeratotic zone of keratinization (→). HE stain, ×200.
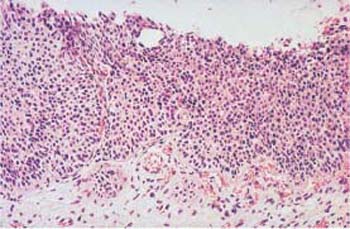
Fig. 4.27 Small-cell carcinoma in situ of the cervical epithelium. The basal cell layer consists of very small immature cells with hyperchromatic, monomorphic nuclei, and it occupies the entire thickness of the epithelium. The uniform size of the cells gives the epithelium a monomorphic character. HE stain, ×200.
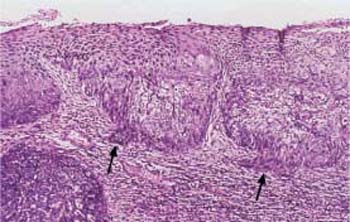
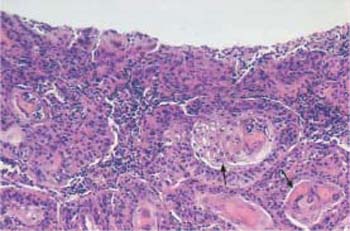
Fig. 4.29 Keratinizing squamous carcinoma of the cervix. Almost all cells show keratinization, either as individual cells or as keratin pearls (→). The variation in cell size gives the abnormal epithelium a polymorphic character. HE stain, ×100.
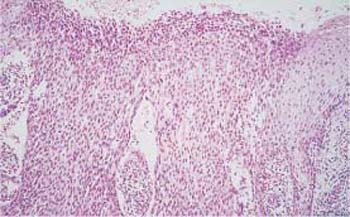
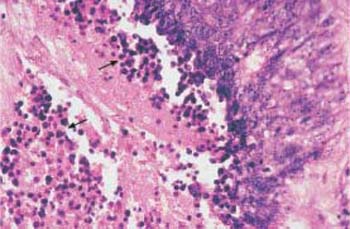
Fig. 4.30 Small-cell squamous carcinoma of the cervix. The abnormal epithelium consists of small cells only and is monomorphic in character. HE stain, ×100.
| Squamous carcinomas |
|
| Adenocarcinomas |
|
| Other epithelial carcinomas |
|
| Sarcomas |
|
| Mixed tumors |
|
| Mixed cell tumors |
|
| Formation of metastases |
The 1994 WHO classification (Table 4.1)no longer distinguishes a small-cell squamous carcinoma (348). Instead, it lists an undifferentiated (anaplastic) small-cell carcinoma, which is of neuroendocrine origin and corresponds to the oat cell carcinoma of the lung. This type of carcinoma is very rare and represents only about 1 % of all cervical carcinomas. Because of the small size of its cells (Fig. 4.31), it is usually not recognized cytologically, thus leading to early onset of metastasis with high mortality. Since the small-cell squamous carcinoma mentioned before (see p. 177) is observed much more often, it may not be wise to abandon the classic nomenclature (309) in favor of the new WHO classification (348). Furthermore, the three established variants of squamous cell carcinoma can be easily verified in the cytological smear.
Cytology
The great success of cervical cytology in early cancer detection is based on the fact that abnormally altered cell nuclei can be assigned to different degrees of cytoplasmic maturation. This makes it possible to distinguish between various grades of dysplasia and to recognize precursors of cervical carcinoma very early on.
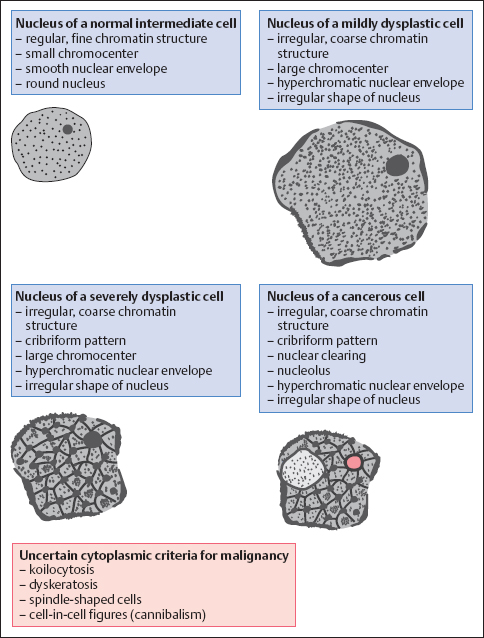
Malignancy Criteria
The morphology of cells exfoliating at the surface of the squamous epithelium makes it possible to determine the degree of severity of the pathological lesion lying underneath. HPV infects exclusively the cell’s nucleus, where it stimulates the replication of DNA and chromosomes. Hence, the criteria for malignancy focus primarily on the nucleus. We distinguish between the following features:
- Abnormal chromatin structure
- Hyperchromatic nuclear envelopes and presence of chromocenters
- Hyperchromatic nuclei
- Polymorphic nuclei (variable size and shape)
- Absolute enlargement of nuclei
- Increased nucleocytoplasmic ratio
- Presence of nucleoli
Abnormal chromatin structure. The most important criterion for malignancy is an irregular, coarse, and therefore abnormal, chromatin structure. The Papanicolaou method uses hematoxylin to stain the nuclear chromatin. The biologically active portion, the euchromatin, appears bright whereas the genetically inactive portion during interphase, the heterochromatin, appears dark. The heterochromatin particles of normal nuclei are finely granular and evenly distributed (Fig. 4.32) (137, 146).
In contrast, abnormal nuclei show an increase in heterochromatin, and this is proportional to the degree of dysplasia. The more severe stages of dysplasia show dark chromatin bands that form bridges between the coarsened granules, thus creating a cribriform pattern—which is nevertheless still fairly regular (116). In invasive carcinoma, however, the nuclei exhibit irregular distribution of the chromatin due to the clumping of heterochromatin. Large bright areas within the chromatin pattern represent active parts of the nucleus and are referred to as nuclear clearings (44).
A coarse chromatin structure needs to be distinguished from degenerative nuclear changes (81). Karyorrhexis is characterized by disintegration of the chromatin bridges described above, leaving behind chromatin fragments (see pp. 57 ff.). This is accompanied by gaps in the nuclear envelope (26).
Hyperchromatic nuclear envelopes and chromocenters. Heterochromatin adhering to the inner nuclear membrane results in a hyperchromatic nuclear envelope of irregular thickness, and clumping of heterochromatin inside the nucleus results in the formation of chromocenters (138). During degenerative and inflammatory processes, on the other hand, the nuclear membrane usually remains smooth despite being thickened, and chromocenters are less prominent (see pp. 57 ff. and 109 ff.).
Hyperchromatic nuclei. This additional sign of malignancy is due to an increased amount of heterochromatin (4). In highly malignant cells with rapid cell division and short interphases, however, euchromatin often exceeds heterochromatin, and the nuclear stain is only faint. Inflammation may also result in hyperchromatic nuclei, and this is sometimes so pronounced that it becomes difficult to distinguish between inflammation and malignancy (368).
Polymorphic nuclei. Another common criterion for malignancy is that the nuclei vary in size and shape, which is called nuclear polymorphism.
Differences in nuclear size result from uncoordinated DNA synthesis; this sign of malignancy is called anisonucleosis (64).
Changes in nuclear shape include loss of roundness, indentations, and lobes. These irregularities result from an increased metabolic exchange between the nucleus and the cytoplasm. The increased metabolism requires an enlarged nuclear surface, which is achieved by folds and indentations of the nuclear membrane. Extreme cytoplasmic invagination creates ring-shaped nuclei.
However, nuclear changes are not necessarily a sign of malignancy as they also occur with degeneration and inflammation.
Absolute nuclear enlargement. This is an important criterion, but not very specific because it is also observed with inflammation (see pp. 109 ff.). In dysplasia, the nuclear area is about three to four times larger than in normal superficial cells, but it is only about twice the size in carcinomas (306). Cells with enlarged nuclei should only be evaluated as malignant when other signs of malignancy are present as well.
Increased nucleocytoplasmic ratio. The ratio of nuclear volume to cytoplasmic volume indicates malignancy if there is a greater shift in favor of the nucleus than is the case with benign processes.
Presence of nucleoli. Another unreliable criterion for malignancy is the formation of nucleoli, a feature also associated with many benign changes, particularly during cell regeneration (see pp.123, 138 ff.) (394). Although nucleoli are essentially absent from dysplastic cells and even from carcinoma in situ, they are regularly found in invasive carcinomas. Hence, their detection in a carcinoma in situ indicates the start of invasion (206). The presence of nucleoli signifies an increase in metabolic activity and protein synthesis in the cell. Since formation of a nucleolus requires a relatively long interphase, nucleoli are not detected in very rapidly growing, anaplastic carcinomas (72).
Reliability of the criteria. None of the criteria for malignancy (Table 4.2) is absolutely reliable, since they occasionally also occur with certain benign processes. The more criteria are detected simultaneously in the cytological smear, the more likely they are to indicate the existence of a malignant lesion. The differential diagnosis between benign and malignant nuclear changes may be very difficult in some cases.
If the cells in the preparation are well preserved and the critical features are very prominent, the diagnosis is relatively simple. If inflammatory or degenerative processes influence the cytological image and the signs are poorly developed, the diagnosis becomes considerably more difficult or even impossible. On the other hand, inflammation and degeneration may cause cellular and nuclear changes so severe that they mimic the criteria for malignancy, thus giving rise to a false positive diagnosis.
Classification Criteria
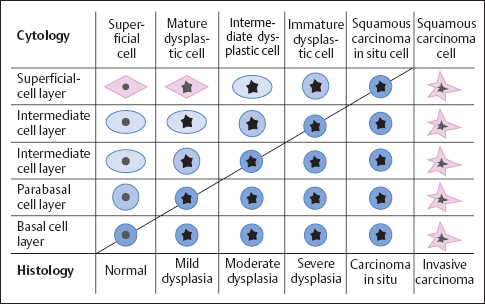
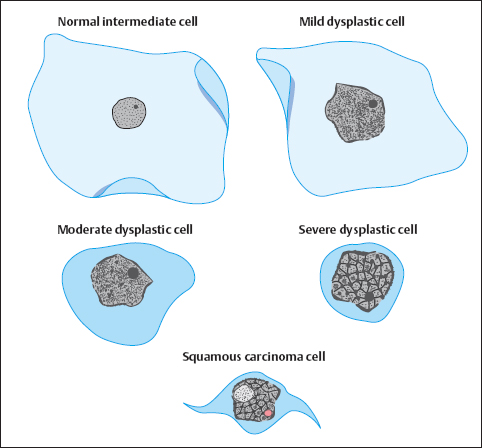
Corresponding to the histologically defined grades of dysplasia that lead to invasive carcinoma, we can cytologically distinguish the following precancerous stages based on the cytoplasmic maturation of dysplastic cells (see also Fig. 4.7, p. 171):
- mild dysplasia
- moderate dysplasia
- severe dysplasia
- squamous carcinoma in situ
- invasive squamous carcinoma
Additional facultative cellular phenomena include:
- Signs of HPV infection
- Cell-in-cell configurations
- Spindle-shaped cells
- Gland-shaped cell clusters
With respect to the morphological origin, we distinguish:
- Nonkeratinizing dysplasia
- Metaplastic dysplasia
- Keratinizing dysplasia
- Small-cell dysplasia
- Radiation-induced dysplasia
- Glandular dysplasia (see p. 218 ff.)
Grades of dysplasia. The term dyskaryosis, which was introduced by Papanicolaou and is partly still in use today, is used when nuclei in the cytological smear meet the criteria for malignancy described earlier. The morphology of the cytoplasm surrounding these nuclei provides the decisive clue to the origin of the dyskaryotic cells, thus permitting classification of the lesion.
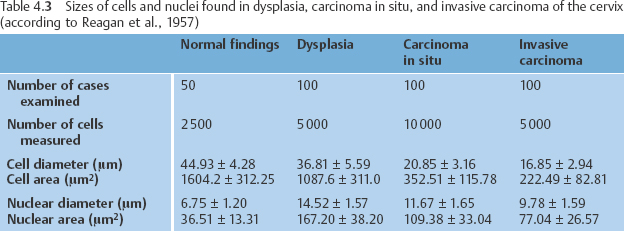
A dyskaryotic cell is a squamous epithelial cell of a certain degree of maturity—or a columnar epithelial cell—that contains an abnormal nucleus (Fig. 4.33) (278). Table 4.3 presents data obtained by measuring cytoplasm and nuclei of abnormal squamous cells associated with various degrees of malignancy. The data reveal that the size of the cells diminishes with increasing malignancy. The size of the nuclei, on the other hand, remains relatively constant, although the nuclei are larger in dysplasia than in carcinoma. Abnormal cells exfoliate as small clusters of cells and sometimes as single cells. In most cases, dysplasia affects squamous epithelia that mature normally and, therefore, leads to nonkeratinizing dysplasia.
Mild dysplasia. This involves superficial or intermediate (mature) cells that show dyskaryosis (Fig. 4.34a–c).
Moderate dysplasia. This involves parabasal (intermediate) cells and that show dyskaryosis (Fig. 4.35a–c) (310, 413).
Severe dysplasia. This involves basal cells that show dyskaryosis (Fig.4.36a, b).
Squamous carcinoma in situ. Monomorphic immature cells showing dyskaryosis and appearing in single-file arrangement (“Indian files”) indicate squamous carcinoma in situ (Figs. 4.37a–c, 4.38) (304).
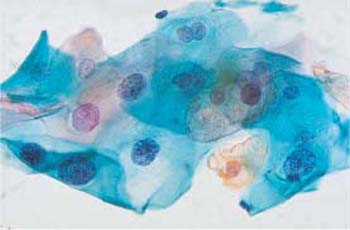
Facultative cellular phenomena. In addition to dysplastic cells, there are occasionally other morphological abnormalities. Koilocytes or other indirect signs of HPV-induced changes are often detected (Figs. 4.39a, b, 4.40a, b) (373). The lower the grade of dysplasia, the more common these are. They are almost never associated with CIN III lesions and invasive carcinomas. The detection of cytological signs of HPV, however, is in no way correlated with the microbiological detection of the pathogen; the latter is several-fold more efficient (393).
A sign frequently observed during the entire process of carcinogenesis is the cell-in-cell configuration (Figs. 4.41a, b, 4.42a, b) (81). Here, individual abnormal squamous cells either have incorporated leukocytes (phagocytosis) or have engulfed other squamous epithelial cells (cannibalism). The foreign cellular elements usually become encapsulated in a cytoplasmic vacuole.
All precancerous stages may occasionally be associated with abnormal spindle-shaped cells appearing in the smear. These are the result of a dysplastic surface effect (Figs. 4.43, 4.44a, b, 4.45a, b) (281).
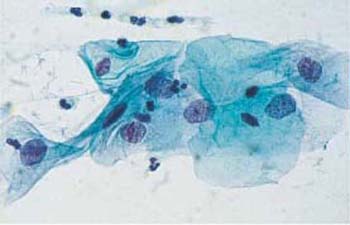
b Superficial and intermediate cells with enlarged, hyperchromatic nuclei and irregular chromatin structure. ×630.
c The cells at the top show enlarged, hyperchromatic nuclei with irregular chromatin pattern, hyperchromatic nuclear envelope, and formation of chromocenters (→). Normal superficial cells are visible at the bottom. ×1000.
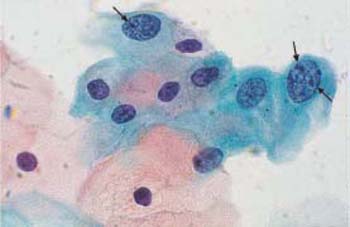
b Small intermediate and parabasal cells with enlarged, abnormal, and partly condensed nuclei. Normal superficial and intermediate cells are visible nearby. ×400.
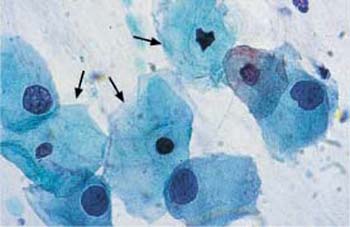
c Small intermediate cells with enlarged, hyperchromatic nuclei and irregular chromatin structure. Normal intermediate cells are also visible →. ×630.
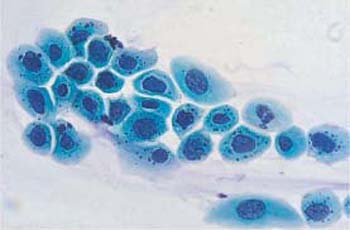
b Parabasal epithelial cells with polymorphic, abnormal, and partly condensed nuclei. A normal intermediate cell is visible in the center. ×1000.
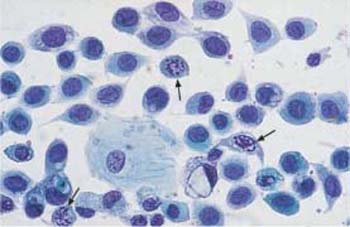
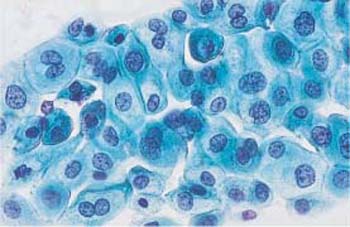
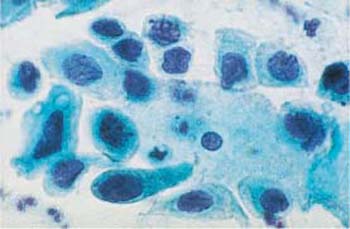
Fig. 4.37a–c Squamous carcinoma in situ cells.
a Fairly uniform abnormal basal cells with irregular, coarse chromatin structure. Some nuclei show karyorrhexis (→). A small normal intermediate cell is visible at the bottom. ×630.
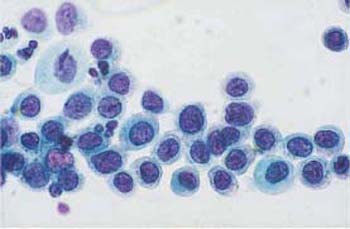
b Abnormal basal cells of uniform morphology. They all show signs of malignancy. ×1000.
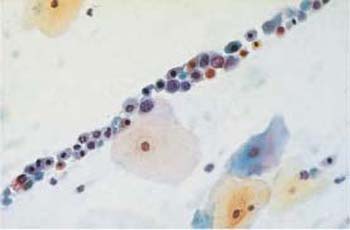
c Characteristic striplike arrangement of abnormal basal cells with partly active, partly degenerated nuclei. Normal superficial cells are visible nearby. ×400.
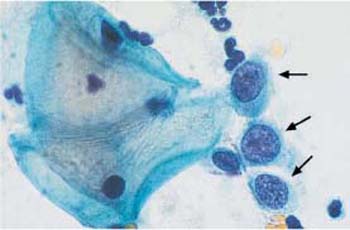
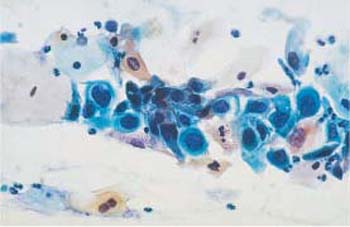
Fig. 4.38 Monomorphic dyskaryosis associated with carcinoma in situ of the cervix. Abnormal basal cells in single file (→), showing irregular chromatin structure and chromocenters. Two normal superficial cells are visible on the left. ×1000.
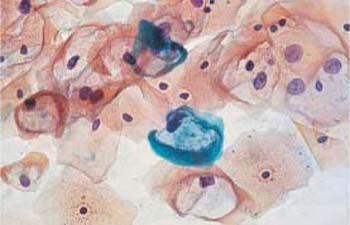
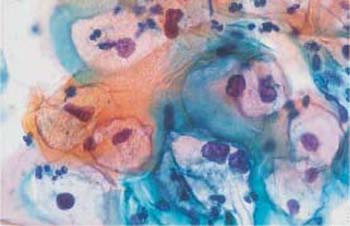
b The nuclei are partly abnormal, and some cells contain two nuclei. ×630.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 ) show an increase in cytoplasm and more defined cell boundaries. HE stain, ×400.
) show an increase in cytoplasm and more defined cell boundaries. HE stain, ×400.