Ingrid Nygaard
Lewis Wall
Physiology of Micturition
The bladder is a complex organ that has a relatively simple function: to store urine effortlessly, painlessly, and without leakage and to discharge urine voluntarily, effortlessly, completely, and painlessly. To meet these demands, the bladder must have normal anatomic support and normal neurophysiologic function.
Normal Urethral Closure
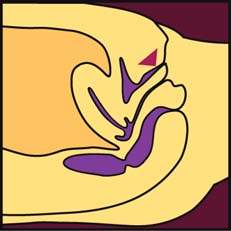
Normal urethral closure is maintained by a combination of intrinsic and extrinsic factors. The extrinsic factors include the levator ani muscles, the endopelvic fascia, and their attachments to the pelvic sidewalls and the urethra. This structure forms a hammock beneath the urethra that responds to increases in intra-abdominal pressure by tensing, allowing the urethra to be closed against the posterior supporting shelf (Fig. 26.1). When this supportive mechanism becomes faulty for some reason—the endopelvic fascia has detached from its normal points of fixation, muscular support has weakened, or a combination of these two processes—normal support is lost and anatomic hypermobility of the urethra and bladder neck develops. For many women, this loss of support is severe enough to cause loss of closure during periods of increased intra-abdominal pressure, resulting in stress incontinence. However, many women remain continent in spite of loss of urethral support (1).
Figure 26.1 Lateral view of the pelvic floor drawn from a three-dimensional reconstruction with the urethra, vagina, and fascial tissues transected at the level of the vesical neck. Note how the urethra is compressed against the underlying supportive tissues by the downward force (arrow) generated by a cough or sneeze. (From Delancey J. Structural support of the urethra as it relates to stress urinary incontinence: the hammock hypothesis. Am J Obstet Gynecol 1994;170:1718, with permission.)
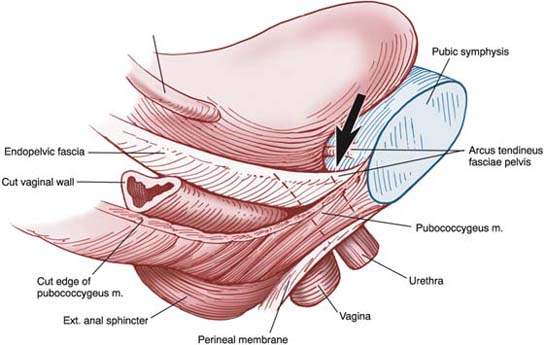
The intrinsic factors contributing to urethral closure include the striated muscle of the urethral wall, vascular congestion of the submucosal venous plexus, the smooth muscle of the urethral wall and associated blood vessels, the epithelial coaptation of the folds of the urethral lining, urethral elasticity, and the tone of the urethra as mediated by α–adrenergic receptors of the sympathetic nervous system.
Effective urethral closure is maintained by the interaction of extrinsic urethral support and intrinsic urethral integrity, each of which is influenced by several factors (muscle tone and strength, innervation, fascial integrity, urethral elasticity, coaptation of urothelial folds, urethral vascularity). In the clinical setting, damaged urethral support is manifested clinically by urethral hypermobility, which often results in incompetent urethral closure during physical activity and presents as stress urinary incontinence. Intrinsic urethral functioning is more complicated and is not understood nearly as well as incontinence related to loss of urethral support (2).
Clinical appreciation of the importance of extrinsic support and intrinsic urethral function led to the separation of stress incontinence into two broad types:
Surgical approaches are based on this arbitrary distinction, with a pubovaginal sling recommended for women with intrinsic sphincter deficiency and a colposuspension (also known as retropubic urethropexy) for those with hypermobility. This rationale was based initially on a small study in which women younger than age 50 years with urethral closure pressure less than 20 cm H2O had a higher failure rate after a Burch colposuspension than did women with a closure pressure greater than 20 cm H2O (3). No difference in outcome was seen in women older than 50 years. This dichotomy was called into question, based on the observation that all women with stress incontinence have some degree of sphincter weakness, regardless of whether they have hypermobility. Minimally invasive synthetic midurethral slings have largely replaced pubovaginal slings and retropubic urethropexy as the most commonly performed surgical procedures for stress urinary incontinence. The use of midurethral slings would seem to lessen the impact of a poorly functioning urethra; however, a similar debate is ongoing about the impact of poor urethral function with both retropubic and transobturator slings. It appears that women with poor urethral function are more likely to experience treatment failure irrespective of the type of procedure performed (4).
The Bladder
The bladder is a bag of smooth muscle that stores urine and contracts to expel urine under voluntary control. It is a low-pressure system that expands to accommodate increasing volumes of urine without an appreciable rise in pressure. This function appears to be mediated primarily by the sympathetic nervous system. During bladder filling, there is an accompanying increase in outlet resistance. The bladder muscle (the detrusor) should remain inactive during bladder filling, without involuntary contractions. When the bladder has filled to a certain volume, fullness is registered by tension-stretch receptors, which signal the brain to initiate a micturition reflex. This reflex is controlled by cortical control mechanisms, depending on the social circumstances and the state of the patient’s nervous system. Normal voiding is accomplished by voluntary relaxation of the pelvic floor and urethra, accompanied by sustained contraction of the detrusor muscle, leading to complete bladder emptying.
Innervation
The lower urinary tract receives its innervation from three sources: (i) the sympathetic and (ii) parasympathetic divisions of the autonomic nervous system, and (iii) the neurons of the somatic nervous system (external urethral sphincter). The autonomic nervous system consists of all efferent pathways with ganglionic synapses that lie outside the central nervous system. The sympathetic system primarily controls bladder storage, and the parasympathetic nervous system controls bladder emptying. The somatic nervous system plays only a peripheral role in neurologic control of the lower urinary tract through its innervation of the pelvic floor and external urethral sphincter.
The sympathetic nervous system originates in the thoracolumbar spinal cord, principally T11 through L2 or L3 (see Chapter 6). The ganglia of the sympathetic nervous system are located close to the spinal cord and use acetylcholine as the preganglionic neurotransmitter. The postganglionic neurotransmitter in the sympathetic nervous system is norepinephrine, and it acts on two types of receptors: α-receptors, located principally in the urethra and bladder neck, and β-receptors, located principally in the bladder body. Stimulation of α-receptors increases urethral tone and thus promotes closure, whereas α–adrenergic receptor blockers have the opposite effect. Stimulation of β-receptors decreases tone in the bladder body.
The parasympathetic nervous system controls bladder motor function—bladder contraction and bladder emptying. The parasympathetic nervous system originates in the sacral spinal cord, primarily in S2 to S4, as does the somatic innervation of the pelvic floor, urethra, and external anal sphincter. Sensation in the perineum is also controlled by sensory fibers that connect with the spinal cord at this level. For this reason, examination of perineal sensation, pelvic muscle reflexes, and pelvic muscle or anal sphincter tone is relevant to clinical evaluation of the lower urinary tract. The parasympathetic neurons have long preganglionic neurons and short postganglionic neurons, which are located in the end organ. Both the preganglionic and postganglionic synapses use acetylcholine as their neurotransmitter, acting on muscarinic receptors. Because acetylcholine is the main neurotransmitter used in bladder muscle contraction, virtually all drugs used to control detrusor muscle overactivity have anticholinergic properties.
Bladder storage and bladder emptying involve the interplay of the sympathetic and parasympathetic nervous systems. The modulation of these activities appears to be influenced by a variety of nonadrenergic, noncholinergic neurotransmitters and neuropeptides, which fine tune the system at various facilitative and inhibitory levels in the spinal cord and higher areas of the central nervous system (5–7). Neuropathology at almost any level of the neurourologic axis can have an adverse effect on lower urinary tract function.
Micturition
Micturition is triggered by the peripheral nervous system under the control of the central nervous system. It is useful to consider this event as occurring at a micturition threshold, a bladder volume at which reflex detrusor contractions occur. The threshold volume is not fixed; rather, it is variable and can be altered depending on the contributions made by sensory afferents from the perineum, bladder, colon, rectum, and input from the higher centers of the nervous system. The micturition threshold is, therefore, a floating threshold that can be altered or reset by various influences.
The spinal cord and higher centers of the nervous system have complex patterns of inhibition and facilitation. The most important facilitative center above the spinal cord is the pontine-mesencephalic gray matter of the brainstem, often called the pontine micturition center, which serves as the final common pathway for all bladder motor neurons. Transection of the tracts below this level leads to disturbed bladder emptying, whereas destruction of tracts above this level leads to detrusor overactivity. The cerebellum serves as a major center for coordinating pelvic floor relaxation and the rate, force, and range of detrusor contractions, and there are multiple interconnections between the cerebellum and the brainstem reflex centers. Above this level, the cerebral cortex and related structures exert inhibitory influences on the micturition reflex. Thus, the upper cortex exerts facilitative influences that release inhibition, permitting the anterior pontine micturition center to send efferent impulses down the complex pathways of the spinal cord, where a reflex contraction in the sacral micturition center generates a detrusor contraction that causes bladder emptying.
A normal lower urinary tract is one in which the bladder and urethra store urine without pain until a socially acceptable time and place arises, at which point voiding occurs in a coordinated and complete fashion. Lower urinary tract disorders include disorders of storage (such as urinary incontinence), emptying (such as urinary hesitancy and retention), and sensation (such as urgency or pain). Current definitions for these disorders are depicted in Table 26.1.
Table 26.1 Classification and Definition of Lower Urinary Symptoms in Women
| I. Abnormal Storage |
| Incontinence (symptom) The complaint of any involuntary leakage of urine |
| Stress urinary incontinence (symptom) The complaint of involuntary leakage on effort or exertion, or on sneezing or coughing |
| Stress urinary incontinence (sign) Observation of involuntary leakage from the urethra, synchronous with exertion/effort, or sneezing or coughing |
| Urgency urinary incontinence (symptom) The complaint of involuntary loss of urine associated with urgency |
| Mixed incontinence Complaint of involuntary loss of urine associated with urgency and also with effort or physical exertion or on sneezing or coughing |
| Continuous urinary incontinence Complaint of continuous involuntary loss of urine |
| Frequency The number of voids per day, from waking in the morning until falling asleep at night |
| Increased daytime urinary frequency Complaint that micturition occurs more frequently during waking hours than previously deemed normal by women (traditionally defined as more than seven episodes) |
| Nocturia Complaint of interruption of sleep one or more times because of the need to micturate (each void is preceded and followed by sleep) |
| Nocturnal enuresis Complaint of involuntary loss of urine that occurs during sleep |
| Urgency Compliant of sudden, compelling desire to pass urine, which is difficult to defer |
| Postural urinary incontinence Compliant of involuntary loss of urine associated with change of body position, for example, rising from a seated or lying position |
| Insensible urinary incontinence Compliant of urinary incontinence where the women has been unaware of how it occurred |
| Coital incontinence Compliant of involuntary loss of urine with coitus. This symptoms might be further divided into that occurring with penetration or intromission and that occurring at organism. |
| Overactive bladder syndrome (OAB) Urinary urgency, usually accompanied by frequency and nocturia, with or without urgency urinary incontinence, in the absence of urinary tract infection or other obvious pathology |
| II. Abnormal Sensory Symptoms |
| Increased bladder sensation Complaint that the desire to void during bladder filling occurs earlier or is more persistent from that previous experienced (differs from urgency by the fact that micturition can be postpone despite the desire to void) |
| Reduced bladder sensation Complaint that the definite desire to void occurs later than that previously experienced, despite an awareness that the bladder is filling |
| Absent bladder sensation Complaint of both the absence of the sensation of bladder filling and a definite desire to void |
| III. Abnormal Emptying |
| Hesitancy Compliant of a delay in initiating micturition |
| Straining to void Complaint of the need to make an intensive effort (by abdominal straining, Valsalva or suprapubic pressure) to initiate, maintain, or improve urinary stream |
| Slow stream Complaint of a urinary stream perceived as slower compared to previous performance or in comparison with others |
| Intermittency Complaint of urine flow that stops and starts on one or more occasions during voiding |
| Feeling of incomplete bladder emptying Complaint that the bladder does not feel empty after micturition |
| Postmicturition leakage Complaint of a further involuntary passage of urine following the completion of micturition |
| Spraying of urinary stream Complaint that the urine passage is a spray or split rather than a single discrete stream |
| Position-dependent micturition Complaint of having to take specific positions to be able to micturate spontaneously or to improve bladder emptying, for example, leaning forward or backward on the toilet seat or voiding in a semistanding position |
| Urinary retention Complaint of the inability to pass urine despite persistent effort |
| From Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) Joint Report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn 2010;29:4–20, with permission. |
Urinary Incontinence
Definitions
Defining urinary incontinence would seem an easy task: women who leak urine must be “incontinent.” A joint report from the International Urogynecological Association and the International Continence Society made recommendations on the terminology of female pelvic floor dysfunction in an attempt to update the definitions by a female-specific approach and clinically based consensus. This report defined incontinence as “the complaint of any involuntary leakage of urine” (8). This definition does not take into account the wide variation in this symptom and the disruption it causes. For example, half of young nulliparous women report occasional minor urine leakage; for most this is neither a bother nor a symptom for which they would seek treatment. At the other extreme, 5% to 10% of adult women have severe leakage daily. These women often dramatically alter their lives because of leakage, curtailing activities, social outings, and intimacy. Many suffer marked deterioration in self-esteem. In between these two extremes lies another one-third of adult women who report leakage at least weekly, but without the same degree of life-altering severity as the women previously noted.
Collectively, these women assume a substantial cost burden. The total annual cost to care for patients with incontinence in the United States is estimated at $11.2 billion in the community and $5.2 billion in nursing homes (9). In the United States, much of this cost is borne directly by women in the form of incontinence pads and excess laundry costs. Despite the burden imposed by leakage, many women do not discuss this symptom with a health care professional. For some women, this is because the leakage does not bother them, whereas others are embarrassed and suffer in silence. Still others do not raise this issue because they mistakenly believe the only treatment option is surgical. It is incumbent on the provider to ask women about leakage.
Studies show that there is little relationship between the volume of urine lost and the distress that it causes a patient (10). The degree to which women are bothered by leakage is influenced by various factors, including cultural values and expectations regarding urinary continence and incontinence. If the leakage is distressing to the patient, evaluation and treatment should be offered. Incontinence can almost always be improved and frequently can be cured, often using relatively simple, nonsurgical interventions.
Types of Disorders
Stress Urinary Incontinence
Stress urinary incontinence occurs during periods of increased intra-abdominal pressure (e.g., sneezing, coughing, or exercise) when the intravesical pressure rises higher than the pressure that the urethral closure mechanism can withstand. Some advocate the term “activity-related incontinence” in some languages to avoid the confusion with psychological stress (8). Stress urinary incontinence is the most common form of urinary incontinence in women and is particularly common in younger women. Active women are more likely to notice symptoms of stress urinary incontinence. In a survey of 144 collegiate female varsity athletes, 27% reported stress incontinence while participating in their sport (11). The activities most likely to produce urinary loss were jumping, high-impact landings, and running.
Stress incontinence is an interesting “disease” as the same symptoms have varying effects on different women. This condition is best considered in a biobehavioral model that examines the interaction of three variables: (i) the biologic strength of the urethral sphincteric mechanism, (ii) the level of physical stress placed on the closure mechanism, and (iii) the woman’s expectations about urinary control. This model explains the enormous variation that exists among the symptoms, the degree of demonstrable leakage, and a patient’s response to her stress incontinence. Modification of any one of these factors may influence the patient’s clinical status; for example, many patients give up certain physical activities (e.g., running, dancing, aerobics) when they experience stress incontinence. Limiting their activities may eliminate the incontinence problem, but it does so at a certain cost to their quality of life. Other women learn to cope with stress incontinence by adopting new body postures during physical activities that prevent them from leaking or by strengthening their pelvic muscles to compensate for increased exertion. Other women may be profoundly relieved to find out that the small amount of leakage they experience from time to time is not abnormal. In any case, the interaction of these three biopsychosocial factors opens up a variety of strategies for the management of stress incontinence. Surgical intervention is only one strategy, and it addresses only the biologic competence of the sphincteric mechanism rather than either of the other factors that interact to produce the clinical problem.
Urgency Urinary Incontinence and Overactive Bladder
Although stress incontinence is the most common type of urinary continence in all women, urgency incontinence is the most common form of incontinence in older women (12). Urgency urinary incontinence is the involuntary leakage of urine accompanied by or immediately preceded by urgency. The new joint report from the International Urogynecological Association and International Continence Society recommended this symptom be called urgency urinary incontinence to differentiate between the normal urge experienced when the bladder is full from the abnormal response that may require treatment. This is a symptom-based diagnosis; the cause may or may not be detrusor overactivity, based on urodynamic observation characterized by involuntary detrusor contractions during the filling phase.
Women may have other related problems such as urgency, nocturia, and increased daytime frequency. The definition of nocturia is quantifiable: The woman wakes one or more times a night to void (8). Increased daytime frequency occurs when the patient considers that she voids too often. The term pollakisuria is used to describe this condition in many countries.
Urgency is the sudden compelling desire to pass urine that is difficult to defer. Most women have experienced these symptoms during times of voluntary delays in voiding or increased fluid intake. However, urinary urgency implies more than just the feeling that all normal women have if they voluntarily delay voiding beyond a reasonable time (8). When a woman presents for treatment, she generally reports an intrusive, bothersome, persistent need to urinate that takes her attention away from other activities. Increased daytime frequency is often brought up as an issue when a woman experiences a change in her own voiding pattern.
There is very little information about what is “normal” in terms of voiding frequency. Overactive bladder (OAB) is frequently defined in studies of pharmacologic agents as more than eight voids per 24 hours, a definition that was based on the 95th percentile of voids in a small sample of Scandinavian women (13). Data from a broader sample of women in the United States suggest that the median number of voids per day is eight, and 95% of so-called normal women void 12 or fewer times per day (14).
Overactive detrusor function is defined as a urodynamic diagnosis characterized by involuntary detrusor contractions during the filling phase, which may be spontaneous or provoked. It is divided into neurogenic detrusor overactivity, resulting from a relevant neurologic condition and idiopathic detrusor overactivity, when there is no clear cause (15).
The term overactive bladder syndrome is defined as urinary urgency, usually accompanied by frequency and nocturia, with or without urgency urinary incontinence, in the absence of urinary tract infection or other obvious pathology (8). It is often referred to as OAB-dry when women with these symptoms do not leak urine, and OAB-wet when it is accompanied by incontinence. It is important to note that a woman with severe urgency and a sense of impending leakage who remains dry may have the exact same bladder pathology as one with severe urgency and concomitant leakage. A woman with a strong urethral sphincteric mechanism may be able to avoid leakage during uninhibited bladder contractions, and although one with a strong sphincter may remain dry, she still may be disturbed by the urgency and impending sense of leakage.
Mixed Incontinence
As implied by the name, women with mixed incontinence have symptoms of both stress and urge urinary incontinence. Younger women are more likely to have stress incontinence alone, whereas in older women mixed and urge incontinence predominate. In a review of 15 population-based studies of women of all ages with urinary incontinence, a median of 49% (range 24% to 75%) had stress urinary incontinence, 21% (range 7% to 49%) had urge urinary incontinence, and 29% (range 11% to 61%) had mixed urinary incontinence (16).
Functional and Transient Incontinence
Functional incontinence is more common in elderly women and refers to incontinence that occurs because of factors unrelated to the physiologic voiding mechanism. A woman who cannot get to the bathroom quickly enough may often become incontinent. Functional incontinence can be related to such factors as decreased mobility, musculoskeletal pain, or poor vision. Factors leading to transient urinary incontinence are, as the name implies, medically reversible conditions. A useful mnemonic to help remember these factors is DIAPPERS (17,18) (Table 26.2). These factors argue strongly for the inclusion of a thorough medical evaluation as part of the workup of any patient with urinary incontinence.
Table 26.2 Reversible Causes of Incontinence
| D Delirium |
| I Infection |
| A Atrophic urethritis and vaginitis |
| P Pharmacologic causes |
| P Psychological causes |
| E Excessive urine production |
| R Restricted mobility |
| S Stool impaction |
| From Resnick NM, Yalla SV. Management of urinary incontinence in the elderly. N Engl J Med 1985;313:800–805, with permission. |
Extraurethral Incontinence
Although most urinary incontinence represents unwanted urine loss through the urethra (transurethral incontinence), urine loss can also occur through abnormal openings. These openings can be created by congenital defects or some form of trauma. The congenital causes of urinary incontinence are not common and usually are easy to diagnose. The most extreme cases are caused by bladder exstrophy, in which there is a congenital absence of the lower anterior abdominal wall and anterior portion of the bladder, resulting in the entire bladder opening directly to the outside (19). Such cases are diagnosed at birth. Before the advent of modern reconstructive surgery, these infants usually died very early in life from sepsis.
Ectopic ureter, a subtle congenital anomaly causing extraurethral urine loss, generally is detected early in life, but occasionally may escape detection until adolescence or early adulthood (20). In infancy, an ectopic ureter should be suspected when a mother seeks care for her baby, whom she says is never dry. Normally, infants have periods of dryness interspersed with periods of wetness. Most commonly, the ectopic ureter drains into the vagina, but occasionally, it may drain into the urethra distal to the point of continence. This condition can be diagnosed by excretory urography.
A traumatic opening between the urinary tract and the outside is called a fistula. Vesicovaginal fistulas, located between the bladder and urethra, are most common, but fistulas may occur between the vagina, uterus, or bowel, and the urethra, ureter, or bladder.
Worldwide, the most common cause of vesicovaginal fistulas is obstructed labor. This was true in the Western world 150 years ago, but advances in the provision of basic obstetric services and advanced obstetric intervention have virtually eliminated this problem in developed countries.
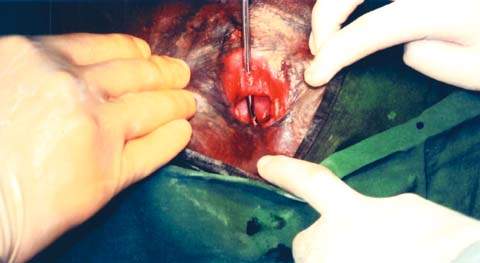
Obstructed labor can occur in rural areas where girls are married young (sometimes as early as 9 to 10 years of age) and where transportation is poor and access to medical services is limited. In such circumstances, pregnancy often occurs shortly after menstruation begins and before maternal skeletal growth is complete. When labor begins, cephalopelvic disproportion is common and little can be done to correct fetal malpresentations. Women may be in labor as long as 5 to 6 days without intervention, and if they survive, they usually give birth to a stillborn infant. In such cases, the soft tissues of the pelvis are crushed by constant pressure from the fetal head, leading to an ischemic vascular injury and subsequent tissue necrosis. When this tissue sloughs, a genitourinary or rectovaginal fistula develops. Many of these patients have complex or multiple fistulas, involving total destruction of the urethra and sloughing of the entire bladder base (Fig. 26.2). Obstetric fistulas are frequently as large as 5 to 6 cm in diameter.
Figure 26.2 Moderate-sized obstetric vesicovaginal fistula. A metal probe has been placed through the urethra and is clearly visible through the bladder base. (Copyright Worldwide Fistula Fund, used by permission.)
After such fistulas develop, the lives of these young women (most of whom are younger than 20 years of age) are ruined unless they can gain access to curative surgical services. The constant, uncontrolled dribble of urine makes them offensive to their husbands and family members and they are often ostracized from their families. Most of them eventually become destitute social outcasts—and yet these are otherwise healthy young women. The social and economic costs of this problem are enormous, yet the world medical community largely ignores it. The morbidity associated with obstetric fistulas remains, along with the related maternal mortality, one of the single most neglected issues in international women’s health care.
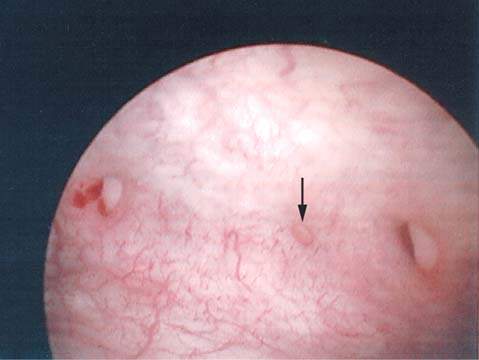
In the industrialized world, the most common causes of genitourinary fistulas are surgery, malignancy, and radiation therapy, alone or in combination. Most often a vesicovaginal fistula develops after an otherwise uncomplicated vaginal or abdominal hysterectomy in which a small portion of the bladder was inadvertently trapped in a surgical clamp or was transfixed by a suture. These fistulas most often occur at the vaginal apex and are no larger than 1 to 2 mm. The amount of urine that can leak through a fistula of any size, however, is enormous. Figure 26.3 shows a cystoscopic view of three small vesicovaginal fistulae, lined up where the cuff suture line would have been. With traditional vaginal and abdominal hysterectomy, many surgeons recommend universal cystoscopy at the completion of the surgical case to assess for urinary tract injury and potentially decrease the incidence of urinary tract fistula. A review of 839 patients undergoing hysterectomy for benign disease followed by universal cystoscopy at completion of the procedure revealed lower urinary tract injury in 4.3%, including bladder injury in 2.9% and ureteral injury in 1.8% (21).
Figure 26.3 Posthysterectomy fistulas. Note three small fistulas in a row.
With the increase in minimally invasive techniques for pelvic surgery, including hysterectomy, the use of electrocautery devices is commonplace. This more frequent use of electrocautery for ligation of vessels and the resulting thermal spread increases concern about the possibility of ureteral damage that may lead to ureterovaginal fistula. When significant urine leakage occurs, often 10 to 14 days following a laparoscopic hysterectomy, ureterovaginal fistula should be strongly considered in the differential diagnosis. As with abdominal and vaginal surgery, careful attention to the location of the ureter, especially in proximity to the uterine arteries, must be a standard precaution. The incidence of ureterovaginal fistula after laparoscopic hysterectomy appears to be 1% to 4% (22).
Although rare, vesicouterine fistulas are increasing in incidence as the rate of cesarean deliveries increases. Such fistulas are almost always associated with repeat cesarean deliveries. The classic triad of vaginal urinary leakage, cyclic hematuria, and amenorrhea is known as Youssef’s syndrome (23).
Nocturia
Nocturia is the number of voids recorded during a night’s sleep; each void is preceded and followed by sleep. To sort out whether nocturia results from heightened urine production at night, the nocturnal urinary volume can be assessed from a bladder chart. Nocturnal urinary volume is defined as the total volume of urine passed between the time the woman goes to bed with the intention of sleeping and the time of waking with the intention of rising. Thus, it excludes the last void before going to bed but includes the first void after rising in the morning. Nocturia can be the result of nocturnal polyuria potentially related to delayed mobilization of fluid especially in the elderly, sleep problems (e.g., sleep apnea), or low volume voids. Nocturnal polyuria is present when an increased proportion of the 24-hour output occurs at night.
Risk Factors for Urinary Incontinence
Most of the data about risk factors for urinary incontinence come from clinical trials or cross-sectional studies using survey design. Some risk factors were more rigorously studied than others. Thus, the information available is limited in its general applicability and one cannot infer causality from it. Despite these limitations, there is some evidence that age, pregnancy, childbirth, obesity, functional impairment, and cognitive impairment are associated with increased rates of incontinence or incontinence severity (16). Some factors pertain more to certain age groups than others. For example, in studies of older women, childbirth no longer increases the risk of incontinence, possibly because of the presence of comorbidities and other factors that promote incontinence. Medical diagnoses that were associated with urinary incontinence include diabetes, strokes, and spinal cord injuries. Other factors about which less is known or findings are contradictory include hysterectomy, constipation, occupational stressors, smoking, and genetics.
Pregnancy and delivery predispose women to stress urinary incontinence, at least during their younger years. Of women who have not borne children, those who are pregnant leak more often than their nonpregnant counterparts; about half of women report symptoms of stress urinary incontinence during pregnancy, but in most, the symptom resolves after delivery. In a prospective study, 32% of 305 primiparas developed stress urinary incontinence during pregnancy and 7% after delivery. By 1 year, only 3% reported stress urinary incontinence (24). However, 5 years later, 19% of women with no symptoms after the first delivery had stress urinary incontinence. Of women reporting stress urinary incontinence 3 months postpartum (in most of whom it had resolved by 1 year), 92% had such leakage 5 years later. Transient postpartum leakage may be a marker for future incontinence.
Various changes happen after delivery that may predispose women to stress urinary incontinence. Levator ani muscle strength decreases (25). About 20% of women develop a visible defect in the levator ani muscles after vaginal delivery (26). The bladder neck descends, and the pelvic muscles undergo partial denervation with pudendal neuropathy (27). In most studies, parity is strongly associated with urinary incontinence in younger women (28). In studies of women 60 years and older, parity is no longer an independent risk factor for incontinence (29). The reason for this is not well elucidated, but it may be because the changes in muscle, nerve, connective tissue, and hormonal function that occur with aging make other women “catch up” to those who developed incontinence at a younger age because of delivery trauma. Alternately, it may be that medical problems more common in older women account for a larger proportion of incontinence risk as women age.
Obesity deserves special mention for its role in causing or exacerbating stress incontinence. Many researchers report an association (that remains after adjusting for age and parity) between increased weight and body mass index (BMI) and urinary incontinence. For example, a dose–response relationship between BMI and severe urinary incontinence was described (30). Compared with women with a BMI less than 25 kg/m2, odds ratios (OR) for the following BMI groups were: 25 to 29, OR 2.0 (range 1.7–2.3); 30 to 34, OR 3.1 (2.6–3.7); 35 to 39, OR 4.2 (3.3–5.3); 40+ 5.0 (3.4-7.3). A prospective randomized study evaluating overweight and obese women with at least 10 urinary incontinence episodes per week undergoing an intensive 6-month weight-loss program versus structured education program found that women in the weight-loss program had a mean weight loss of 8.0% and decreased their leakage episodes by 47% compared to mean weight loss of 1.6% and 28% reduction in leakage episodes in the education program group (31).
Initial Evaluation
The initial evaluation of patients with incontinence requires a systematic approach to consider possible causes. The basic evaluation should include the following items: history (including assessment of quality of life and degree of bother from symptoms), physical examination, and simple primary care level tests. Most women can begin nonsurgical treatment after this basic evaluation.
History
A thorough medical history should be obtained from every incontinent patient. The history should include a review of symptoms, general medical history, review of past surgery, and current medications. The woman’s most troubling symptoms must be ascertained—how often she leaks urine, how much urine she leaks, what provokes urine loss, what improves or worsens the problem, and what treatment (if any) she had in the past. It is essential to keep the patient’s chief symptom at the forefront to avoid inappropriate management. Consider, for example, a woman whose chief concern is that once a month, while leading a business seminar, she has a sudden, overwhelming urge to void followed by complete bladder emptying. She finds this leakage devastating and is considering quitting her job because of her acute embarrassment. On occasion, she leaks a few drops of urine during exercise, but this minor leakage does not bother her. During the evaluation, urodynamics reveal minimal stress urinary incontinence at capacity during strong coughing. No detrusor overactivity is seen. The patient is offered, and undergoes, a surgical procedure for her documented urodynamic stress incontinence. Not surprisingly, her chief symptom is not improved and she is devastated.
The general medical history may reveal systemic illnesses that have a direct bearing on urinary incontinence, such as diabetes mellitus (which produces osmotic diuresis if glucose control is poor), vascular insufficiency (which can lead to incontinence at night when peripheral edema is mobilized into the vascular system, resulting in increased diuresis), chronic pulmonary disease (which can lead to stress incontinence from chronic coughing), or a wide variety of neurologic conditions that can affect the neurourologic axis at any point from the cerebral cortex to the peripheral nervous system. Medications that may affect the lower urinary tract are summarized in Table 26.3 (32–35).
Table 26.3 Medications that May Affect the Urinary Tract
1. Sedatives such as the benzodiazepines may cause confusion and secondary incontinence, particularly for elderly patients. 2. Alcohol may have similar effects to benzodiazepines and also impairs mobility and causes diuresis. 3. Anticholinergic drugs may impair detrusor contractility and may lead to voiding difficulty and overflow incontinence. Drugs with anticholinergic properties are widespread and include antihistamines, antidepressants, antipsychotics, opiates, antispasmodics, and drugs used to treat Parkinson’s disease. 4. α-Agonists, which are often found in over-the-counter cold remedies, increase outlet resistance and may lead to voiding difficulty. 5. α-Blockers, sometimes used to treat hypertension (e.g., prazosin, terazosin), may decrease urethral closure pressure and lead to stress incontinence. 6. Calcium-channel blockers may reduce bladder smooth muscle contractility and lead to voiding problems or incontinence; they may also cause peripheral edema, which may lead to nocturia or nighttime urine loss. 7. Angiotensin-converting enzyme inhibitors may result in a chronic and bothersome cough that can result in increasing stress urinary incontinence in an otherwise asymptomatic patient. |
Quality-of-Life Measures
Physicians caring for incontinent women should ask them about the way the incontinence specifically affects their lives and to what degree the incontinence bothers them. There often is discord between the objective symptom severity and subjective bother. Only by understanding each woman’s situation can treatment be appropriately planned and response evaluated. Some women may be completely satisfied if they are able to sit through a movie without running to the bathroom, even if they leak urine at other times. Others may be satisfied only if they are 100% dry. Given that the latter is likely an unrealistic goal, knowing that the patient feels this way gives the provider the opportunity to educate her about the likely outcome of treatment.
Physicians may use one of several well-designed, validated quality-of-life measures. An expert summary of the literature in this area conducted for the International Consultation on Incontinence recommended the instruments summarized in Table 26.4. These instruments were found to be valid, reliable, and responsive to change following standard psychometric testing.
Table 26.4 Questionnaires to Assess Urinary Incontinence
| The following questionnaires have been recommended by the International Consultation on Incontinence to assess symptoms of incontinence and impact of incontinence on quality of life in women.a |
| Symptoms |
| Urogenital Distress Inventory. Shumaker SA, Wyman JF, Uebersax JS, et al. Health-related quality of life measures for women with urinary incontinence: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Qual Life Res 1994;3:291–306. |
| Urogenital Distress Inventory (UDI)-6 (short form). Uebersax JS, Wyman JF, Shumaker SA, et al. Short forms to assess life quality and symptom distress for urinary incontinence in women: the incontinence impact questionnaire and the urogenital distress inventory. Neurourol Urodyn 1995;14:131–139. |
| Urge-UDI. Lubeck DP, Prebil LA, Peebles P, et al. A health related quality of life measure for use in patient with urge urinary incontinence: a validation study. Qual Life Res 1999;1999:337–344. |
| King’s Health Questionnaire. Kelleher CJ, Cardozo LD, Khullar V, et al. A new questionnaire to assess the quality of life of urinary incontinent women. Br J Obstet Gynaecol 1997;104:1374–1379. |
| Incontinence Severity Index. Sandvik H, Hunskaar S, Seim A, et al. Validation of a severity index in female urinary incontinence and its implementation in an epidemiological survey. J Epidemiol Community Health 1993;47:497–499. |
| Quality of Life |
| Quality of life in persons with urinary incontinence (I-QOL). Wagner TH, Patrick DL, Bavendam TG, et al. Quality of life of persons with urinary incontinence: development of a new measure. Urology 1996;47:67–72. |
| Incontinence Impact Questionnaire. Wyman JF, Harkins SW, Taylor JR, et al. Psychosocial impact of urinary incontinence in women. Obstet Gynecol 1987;70:378–381. |
| Incontinence Impact Questionnaire (IIQ)-7 (short form). Uebersax JS, Wyman JF, Shumaker SA, et al. Short forms to assess life quality and symptom distress for urinary incontinence in women. The incontinence impact questionnaire and the urogenital distress inventory. Neurourol Urodyn 1995;14:131–139. |
| Urge-IIQ. Lubeck DP, Prebil LA, Peebles P, et al. A health related quality of life measure for use in patient with urge urinary incontinence: a validation study. Qual Life Res 1999;1999:337–344. |
aDonovan JL, Badia X, Corcos J, et al. Symptom and quality of life assessment. In: Abrams P, Cardozo L, Khoury J, et al., eds. Incontinence. Plymouth, UK: Plymbridge Distributors, 2002. |
Physical Examination
The physical examination of the patient with incontinence should focus on general medical conditions that may affect the lower urinary tract and problems related to urinary incontinence. Such conditions include cardiovascular insufficiency, pulmonary disease, occult neurologic processes (e.g., multiple sclerosis, stroke, Parkinson’s disease, and anomalies of the spine and lower back), abdominal masses, and mobility. Key factors to assess during the physical examination are summarized in Table 26.5. A cotton swab test has poor predictive value for determining either stress urinary incontinence diagnosis or predicting treatment success (36). It is used by some clinicians to determine movement of the anterior vaginal wall with Valsalva. A woman with a fixed nonmobile urethra is a poor candidate for a surgery (such as a Burch colposuspension) designed to elevate the urethra. It is not possible to increase support for an already well-supported urethra.
Table 26.5 Physical Examination of a Woman with Lower Urinary Tract Dysfunction
| Neurologic |
| Mental status |
| Perineal sensation |
| Perineal reflexes |
| Patellar reflexes |
| Abdominal examination |
| Masses |
| Cardiovascular |
| Congestive heart failure |
| Lower extremity edema |
| Mobility |
| Gait assessment |
| Pelvic examination |
| Prolapse |
| Atrophy |
| Levator muscle palpation (symmetry, ability to squeeze) |
| Anal sphincter function |
| Test of urethral mobility (e.g., cotton swab test) |
Simple (Primary Care Level) Tests
It is important to realize that formal urodynamics tests are neither the only nor the most important tests of bladder function. Other simple tests that can be performed in the primary care setting provide useful information to guide patient care.
Voiding Diary
A frequency/volume bladder chart (often termed a “bladder diary”) is an invaluable aid in the evaluation of patients with urinary incontinence. A frequency/volume chart is a voiding record kept by the patient for several days. Patients are instructed to write down the time of every void on the chart and measure the amount of urine voided. The time of any incontinent episodes, and the specific activities associated with urine loss, should be recorded. If desired, the patient can be instructed to keep a record of fluid intake. Although the type of intake may guide management suggestions, in most cases volume of intake can be estimated with some accuracy from the amount of urine produced.
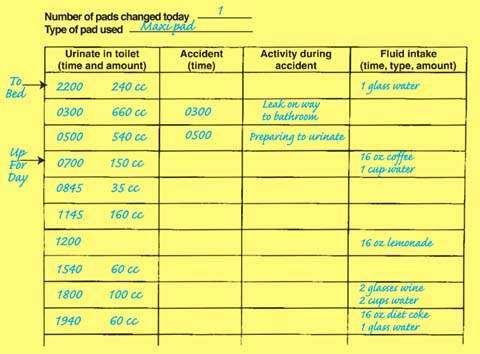
A frequency/volume bladder chart provides vital information about bladder function that is not provided by formal urodynamics studies: 24-hour urinary output, the total number of daily voids, number of nighttime voids, the average voided volume, and the functional bladder capacity (largest volume voided in normal daily life). This information allows the clinician to confirm reports of urinary frequency with objective data and to determine whether part of the patient’s problem is an abnormally high (or low) urinary output. The chart can be used to calculate the volume of urine generated in nighttime hours versus daytime hours. Nighttime volume is calculated by adding output from voids that occur after the woman has fallen asleep for the night and the first morning void on awakening for the day. Older women sometimes have a marked shift in urine production, with more than half of their urine output generated during sleeping hours (Fig. 26.4). Demonstrating this on the voiding diary may lead to further treatment options.
Figure 26.4 Voiding diary (also called bladder chart). Daytime frequency is seven. The patient has nocturia (gets up to void two times during sleeping hours) and also has nocturnal polyuria (an increased proportion of the 24-hour output occurs at night; note that nighttime urine output excludes the last void before sleep but includes the first void of the morning). She has urge incontinence, likely caused by the relatively larger bladder volumes voided at night, which in turn may be related to her greater fluid, caffeine, and alcohol consumption in the evening.
Urinalysis
Examination of the urine by dipstick testing and microscopy is done to exclude infection, hematuria, and metabolic abnormalities. Hematuria cannot be diagnosed on the results of a dipstick test alone; confirmation by microscopic evaluation is mandatory.
If a urinary tract infection is documented by microscopy or culture, it is reasonable to see whether urinary tract symptoms improved with eradication of bacteriuria. Occasionally, a simple urinary tract infection causes the onset or exacerbation of urinary incontinence. Some women, particularly older ones, have asymptomatic bacteriuria that truly is asymptomatic; thus, if attempted treatment of a woman with bacteriuria but without classic urinary tract infection symptoms (such as dysuria, urgency, or frequency) does not improve incontinence, further antibacterial treatment is generally unnecessary.
If hematuria and bacteriuria are found, the urine should be rechecked after eradication of the bacteriuria. Hematuria found in the absence of bacteriuria may need further evaluation to rule out kidney or bladder tumors; the necessity for and extent of the evaluation depends on concomitant risk factors and the clinical presentation. If malignancy is suspected, bladder biopsy should be performed by the surgeon who would treat the patient in the event a malignancy is discovered.
Routine urinary cytology is not helpful, but testing may be of value in women older than 50 years with irritative urinary tract symptoms, particularly if those symptoms are of sudden onset.
Postvoid Residual Volume
Incomplete bladder emptying may cause incontinence. Patients with a large postvoid residual (PVR) urine volume have a diminished functional bladder capacity because of the dead space occupied in the bladder by retained urine. This stagnant pool of urine is a source of urinary tract infections because the major defense of the bladder against infection is frequent, nearly complete emptying.
A large PVR volume can contribute to urinary incontinence in two ways. If the bladder is overdistended, increases in intra-abdominal pressure can force urine past the urethral sphincter, causing stress incontinence (sometimes termed “overflow incontinence” in the context of a large PVR volume). In some cases, bladder overdistention may provoke an uninhibited contraction of the detrusor muscle, leading to incontinence. These conditions may coexist, further complicating the problem.
The PVR volume can be assessed by either direct catheterization or ultrasonography. Although sufficiently accurate for clinical purposes, ultrasonography measurements of PVR volume have a standard error of 15% to 20%. It is reasonable to confirm an elevated PVR volume detected on ultrasound with a catheterized volume (37). It is important to perform this test within 10 minutes of a void to avoid an artificially elevated result because of diuresis. It is agreed that a PVR level less than 50 mL is normal and greater than 200 mL is abnormal, but there is much debate about values in the midrange. Because many women are unable to void well during an anxiety-ridden first visit, it is helpful to recheck the PVR volume at a future visit before embarking on further diagnostic tests. The value of assessing bladder emptying in neurologically normal women who do not have pelvic organ prolapse or symptoms of voiding dysfunction has not been demonstrated.
Cough Stress Test
Patients should be examined with a full bladder, particularly if stress incontinence is a consideration. Urine egress from the urethra at the time of a cough documents stress incontinence. If leakage is not observed when the woman is supine, she should stand with her feet separated and cough several times.
Pad Tests
Weighing menstrual or bladder pads before and after activity provides another objective way to measure urinary leakage. Such pad tests are widely used in patient-oriented research to assess treatment effectiveness, but rarely are they used in clinical practice. Pad tests can be divided into short-term tests, usually performed under standardized office conditions, and long-term tests, usually performed at home for 24 to 48 hours. Pad tests are generally performed with a symptomatically full bladder or with a certain volume of saline instilled into the bladder before beginning the series of exercises. A pad weight gain of 1 g or more is considered positive for a 1-hour test, and a pad weight gain greater than 4 g is positive for a 24-hour test.
Advanced Testing
Urodynamics
At its most basic level, a urodynamic study is anything that provides objective evidence about lower urinary tract function (38). In this sense, measurement of a patient’s voided urine volume and catheterization to determine her PVR volume are urodynamic studies. A frequency/volume chart is also a valuable urodynamic study. Obtaining clinically valuable information does not always require the use of expensive, complex technology. After basic testing, further testing is recommended in the following circumstances: the diagnosis is uncertain (for example, because of major discrepancies between the history, the voiding diary, and symptom scales); surgery is being considered; an elevated PVR volume, a neurologic condition that may complicate treatment (such as multiple sclerosis), marked pelvic organ prolapse, or numerous prior surgical attempts at correction. Bladder and kidney imaging should be considered if the patient has hematuria in the absence of an infection. Current urodynamic definitions are summarized in Table 26.6.
Uroflowmetry
To assess voiding function, urodynamic testing usually begins with uroflowmetry, a study in which the volume of urine voided is plotted over time. Flow time, peak flow rate, and time to peak flow usually increase as the voided volume increases.
Filling Cystometry
Cystometry is done to assess bladder and urethral function during bladder filling. Simple (or single-channel) cystometry is performed when bladder pressure only is measured during filling. Because the bladder is an intra-abdominal organ, the pressure recorded in the bladder is a combination of several other pressures, most notably the pressure created by the activity of the detrusor muscle itself and the pressure exerted on the bladder by the weight of the surrounding intra-abdominal contents (e.g., uterus, intestines, straining, or exertion). For this reason, the technique of complex (also called multichannel or subtracted) cystometry is used to try to approximate the actual pressure exerted in the bladder by the activity of the detrusor muscle alone. The detrusor pressure (Pdet) is obtained by measuring total intravesical pressure (Pves) with a bladder pressure catheter, approximating intra-abdominal pressure (Pabd) with a rectal or vaginal catheter, and then electronically subtracting the latter from the former:
Pdet = Pves – Pabd.
Measurements can be obtained using electronic microtip transducer pressure catheters, fluid-filled pressure lines, fiberoptic catheters, or air-charged catheters. All are acceptable for clinical use, but it is important to realize that when different types of catheters are used, the correlation between numbers is imperfect. Other technical factors that influence cystometry results include the choice of distending medium, filling rate, and patient position. The steps involved in a multichannel urodynamic study are outlined in Table 26.7. Normal cystometric values for women are shown in Table 26.8.
Table 26.7 Steps in Conducting a Multichannel Urodynamic Study
1. Insert pressure and filling catheter into bladder (may be two catheters or dual catheter) to measure intravesical pressure and to fill bladder. Insert pressure catheter into upper vagina or rectum to approximate abdominal pressure. < div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|