Fig. 4.1
Anteroposterior (AP) radiograph of the knee, demonstrating osteosarcoma of the proximal tibia in an 11 year-old
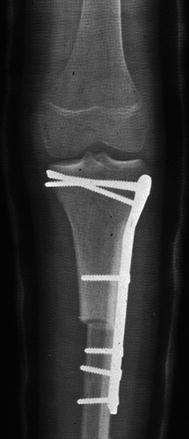
Fig. 4.2
The same patient as in Fig. 4.1, following resection of the proximal tibial osteosarcoma and reconstruction with an osteoarticular allograft
In 1995, Alman and colleagues reported a review of 26 pediatric and adolescent patients who underwent tumor resection and massive allograft reconstruction. Eighteen of the 26 patients had an excellent or good result at final follow-up, and 88 % of patients with a lower extremity reconstruction that survived their disease retained their allograft at final follow-up. However, complication rates were high. At least one complication occurred in 77 % of the patients (not including limb-length discrepancy). Allograft fractures occurred in 54 %, and the infection rate was 12 %. Two of the three patients who developed infection went on to amputation. Four patients (15 %) developed a non-union; each was treated with an additional autologous bone grafting procedure, and each went on to heal within 12 months. A limb-length discrepancy over 2 cm occurred in 60 % of patients with lower extremity reconstruction. The authors concluded that, although the complication rate was higher than in adults, allograft reconstruction was a useful option in younger patients in whom limb-length discrepancy would be predicted to be mild, or those discrepancies that could be easily treated [2].
More recently, Musculo and colleagues published a series of 22 patients under the age of ten who had massive allografts placed after sarcoma resection (13 intercalary and 9 osteoarticular). At latest follow-up, three patients died of their disease, one patient had a subsequent amputation, and 18 patients were alive and had retained their limb. Fifteen of those 18 retained their original allograft. Eight complications occurred which required repeat surgeries (3 local recurrences, 3 fractures, 1 infection, and 1 nonunion); 4 of the 8 had their original allograft preserved. Mean Musculoskeletal Tumor Society (MSTS) functional scores and International Society of Limb Salvage radiographic scores were 27 % and 94 %, respectively. Four patients that had both physes preserved had no limb length discrepancy at final follow-up. Fourteen patients that had resection of one physis with their tumor had a mean leg length discrepancy of 2.1 cm at latest follow-up. The authors therefore concluded that allograft reconstruction was an acceptable technique in young children with extremity sarcoma [37].
4.4.2 Osteoarticular Allografts
Osteoarticular allografts tend to do less well than intercalary allografts, and this is generally due to degeneration of the allograft articular surface secondary to chondrocyte cell death and subchondral bone resorption and collapse [23, 35]. Musculo and colleagues reported on a series of 76 adult and pediatric patients with a tumor of the distal femur that underwent a resection and reconstruction with a distal femoral osteoarticular allograft. Overall allograft survival was 78 % at both 5 and 10 years, and the rate of allograft survival without the need for joint resurfacing was 71 % at both 5 and 10 years. However, 35 % of patients did have radiographic evidence of joint degeneration [38].
In another large series of osteoarticular allografts, Mnaymneh and co-authors reported on a series of 96 patients (adult and pediatric) who underwent distal femoral osteoarticular allograft reconstruction after resection of benign or malignant tumors. Overall complications included a fracture rate of 14 %, nonunion rate of 12 %, and infection rate of 6 %. Clinically significant arthritis developed in 10 %, and instability in 7 %. Significant differences were seen in the results based upon whether or not the patient had received chemotherapy. The overall complication rate in the chemotherapy group was 47 %, compared to 30 % in those patients who did not undergo chemotherapy. In patients who had received chemotherapy, the infection rate was 13 % (versus 2 % without chemotherapy), and the nonunion rate was 23 % (versus 6 %). The modified Mankin classification system was utilized to grade the functional results. Of the patients who did not receive chemotherapy, 70 % had good or excellent results, 26 % had fair results, and 4 % had poor results. In contrast, for those patients who underwent chemotherapy, only 53 % had good or excellent results, 37 % had fair results, and 10 % had poor results. Although the complication rates were high, the authors felt that osteoarticular allograft reconstruction was a viable option, in which most of the complications could be treated adequately [35].
4.4.3 Autografts
While the aforementioned studies, and others, show that massive allografts are useful in pediatric bone sarcoma reconstructions, these procedures are associated with relatively high complication rates. The three most common complications associated with massive allograft reconstruction include infection, nonunion, and fracture. These complications are likely related to the avascular status of the allograft bone [36]. In an effort to reduce these complication rates, free vascularized fibula autografts have been used to reconstruct segmental defects after sarcoma resection. The vascularized fibula graft brings well-perfused bone to the site that is capable of osteogenesis. However, being of smaller diameter, it usually lacks the mechanical strength of large allografts. The fibular autograft will grow, hypertrophy, and remodel over time, but this takes a considerable amount of time, and repeated stress fractures may require repetitive prolonged immobilization [36].
Chen and colleagues from the Memorial Sloan Kettering Cancer Center reported on a series of 25 consecutive patients (adult and pediatric) treated with a vascularized fibular free flap after limb-sparing resection of extremity sarcomas. All flaps survived to final follow-up. The infection rate was 12 %. Uncomplicated bony union occurred in 78 % within 6 months. After secondary bone grafting procedures, 93 % ultimately went on to union. According to MSTS functional scores, all patients who went on to union had good functional results. Eight patient (32 %) developed local recurrence or metastasis, and ultimately six died of their disease. Two of the pediatric patients in the series went on to develop significant leg length discrepancies, both of which had successful limb-lengthening procedures. Based on these results, including lower infection and nonunion rates, the authors recommended vascularized fibular autografts as the reconstruction of choice for long segmental bone defects after tumor resection [11].
4.4.4 Allograft/Autograft Combination
In order to combine the mechanical advantages of massive allograft with the biologic advantages of a vascularized fibula autograft, Capanna and colleagues described a technique of reconstruction that utilizes both a large structural allograft in conjunction with a vascularized fibula [9]. Moran and colleagues from the Mayo Clinic reported on a series of seven pediatric and adolescent patients with extremity sarcoma who were treated with limb salvage using this reconstructive technique (Figs. 4.3, 4.4a, b, and 4.5). The mean follow-up time was 36 months. All seven patients retained their limb at final follow-up. Fibular autograft healing occurred by an average of 4 months, while allograft –host healing tended to take longer, and averaged 9 months. Two cases required secondary bone grafting procedures to obtain union at the allograft-host junction. There were no infections noted during the study period. Two patients developed allograft fractures over 2 years after primary surgery. Both fractures were successfully treated with internal fixation (one also had bone grafting at the time of fracture repair). Four patients had significant limb length discrepancies – two patients with discrepancies less than 2.5 cm were treated with shoe lifts, while two patients with larger discrepancies were treated with contralateral epiphysiodesis and limb shortening procedures. According to the modified Mankin functional classification, there were four excellent results and three good results. These results led the authors to conclude that the Capanna technique is a reliable reconstructive option, and is especially well-suited for the younger patient, whose higher activity demands and longer life span make allograft fracture and infection a more likely issue [36].
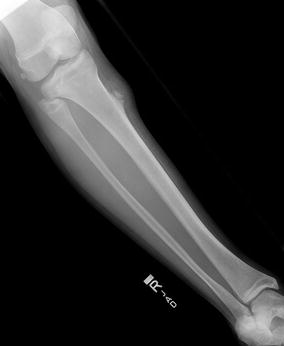
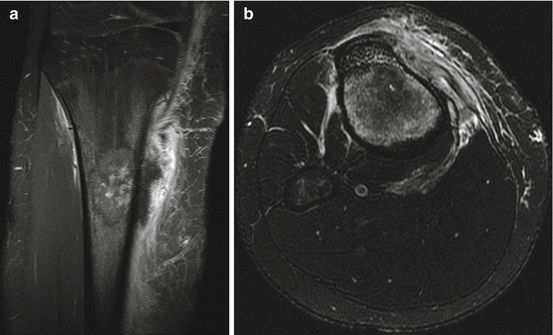

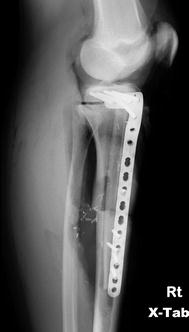
Fig. 4.3
AP x-ray of the tibia, showing a lytic lesion in the proximal tibia of a 14-year-old female with soft tissue extension. Biopsy showed osteosarcoma

Fig. 4.4
(a, b) Coronal (a) and axial (b) MRI images of the patient in Fig. 4.3 following biopsy, showing medullary involvement and soft tissue extension
Cañadell and San-Julian have described an innovative technique that can improve the achievement of a safe surgical margin while helping to preserve the native joint surface in certain appropriately selected children. Most pediatric bone sarcomas occur in the metaphysis, but the physis can serve as a physical barrier to tumor spread. Cañadell and San-Julian have been able to achieve preservation of the epiphysis by performing epiphysiolysis with an external fixator to provide distraction across the physis of 1–1.5 mm per day. This can be performed while the child is receiving his or her neoadjuvant chemotherapy. High quality imaging is required to determine the exact margins of tumor extension [8].
4.5 Prosthetic Reconstruction
Non-biologic reconstruction after resection of an extremity sarcoma involves the placement of a prosthesis to replace the resected bone. The advantages of this technique include better initial fixation which can allow earlier weight-bearing, more predictable function, and lower risk of early complications [1]. Current implants incorporate a modular design, which allows the prosthesis to be assembled to match the defect created at the time of surgery. Additionally, a variety of stem lengths and diameters allow for greater intraoperative flexibility, with cemented and uncemented designs available. Prosthetic designs exist to replace the proximal humerus, proximal femur, distal femur, entire femur, and proximal tibia.
4.5.1 Standard Prostheses
In older children, who are nearing the end of their skeletal growth, a standard, static, adult-type endoprosthesis can be employed, if the expected leg-length discrepancy will be less than 2–3 cm [1]. Additionally, in tumors that occur about the knee, some growth can still be obtained from the remaining physis (i.e. the proximal tibia in distal femur tumors, and vice versa), by placing an uncemented component through a central hole in the physis that would allow the prosthetic stem to slide as the bone continues to grow around it. The distal femoral or proximal tibial physis can then continue to grow without angular deformity, but at a slower rate. The proximal tibia can achieve approximately 80 % of normal growth compared to the contralateral leg, whereas the distal femur can achieve about 60 % [1, 18].
Standard non-expandable endoprostheses have been used for several decades in adult patients, and have also been utilized in pediatric patients who are nearing skeletal maturity. They have a very good overall track record. In a review of 25 patients (adult and pediatric) treated with a proximal femoral endoprosthetic hemiarthroplasty, the 10-year prosthesis survival rate was 86 %. There was one deep infection that was successfully treated with irrigation and debridement and retention of the components; one prosthetic dislocation; and one local recurrence. Three patients had significant acetabular wear, and were planning to undergo acetabular replacement. In each case, the abductor tendons were affixed to the prosthetic trochanter. Some degree of Trendelenburg limp was present in 92 % of patients (mild, 56 %; moderate 16 %; severe, 20 %). Functional outcome was excellent or good in 68 %, fair in 28 %, and poor in 4 % [14].
4.5.2 Expandable Prostheses
Early endoprostheses were static devices that did not allow for lengthening or growth through the device itself. In order to minimize significant limb length discrepancies in younger patients, subsequent procedures were often required to either lengthen the ipsilateral extremity (i.e. distraction osteogenesis), or to slow the growth of or even shorten the contralateral extremity (i.e. epiphysiodesis, shortening osteotomy). In the 1970s and 1980s, endoprosthetic devices began to incorporate designs that would allow lengthening of the prosthesis itself to allow the affected limb to keep pace with the contralateral limb. The first advance that allowed lengthening was the introduction of modularity. A modular prosthesis could be lengthened by exchanging a mid-body segment of the prosthesis for a longer segment, without the need to revise the entire prosthesis. However, this expansion would require a sizable operative exposure, with excision of the pseudocapsule that forms around the prosthesis, in order to perform this type of exchange lengthening. Complications including neurovascular injury, joint stiffness, and infection were not uncommon with these procedures [15].
Later expandable prostheses incorporated designs that would allow for less invasive lengthening procedures over time. These designs worked via one of several mechanisms to allow lengthening, including exchangeable C-collars, ball-bearings, or worm drives. With several of these newer prostheses, expansion procedures could be performed in a percutaneous fashion through a stab incision under fluoroscopic guidance, whereby a screwdriver could be inserted into the lengthening mechanism to expand the prosthesis [1, 4, 6, 15, 18, 40].
In 2000, Eckardt and colleagues published a series of 32 endoprostheses that were implanted following malignant bone tumor resection, which could be lengthened by exchanging modular mid-body segments. Over the 12 years that encompassed the study period, four different prosthesis designs were employed. At the time of publication, half of the patients had undergone at least one lengthening exchange procedure. In the 16 patients that had at least one lengthening procedure, a total of 32 lengthening procedures were performed. Eighteen of the original 32 patients had a total of 27 complications. These complications included aseptic loosening (5 patients), temporary nerve palsy (4 patients), prosthesis collapse or mechanical failure (6 patients), and local recurrence (2 patients). There were no infections noted. Three patients required an operative intervention for knee flexion contractures [15].
Proximal tibia replacements are often associated with even higher rates of complications, most often due to problems with wound breakdown and infection, and due to the challenges in restoring the extensor mechanism. The group from Birmingham, England reported on a series of expandable proximal tibial prostheses in 20 patients in 2000. Five patients died of their disease, and four others underwent above-knee amputation for complications (2 for local recurrence and 2 for infection). There were seven infections, of which five seemed directly related to open lengthening procedures. The authors determined the risk of infection to be 5.1 % per lengthening procedure, and at 10 years the overall risk of infection was 68 %. The patients in the study underwent an average of ten operations, from initial biopsy and prosthesis implant, to lengthenings and procedures to address complications (i.e. knee and ankle manipulations, contracture releases, periprosthetic fractures). However, at final analysis, the average MSTS score was 83 %, and the mean leg length discrepancy was 10 mm. Most patients had a mild to moderate extensor lag, as well as some limitation in knee flexion, which is not dissimilar from the results found in proximal tibial endoprosthetic reconstruction in adults [18].
Even with the development of percutaneous lengthening techniques, patients still required anesthesia, and the multiple operative procedures increased the risk of infection. In order to obviate the need for repeated surgical procedures, the newest expandable endoprosthetic models have incorporated non-invasive lengthening mechanisms [1, 5, 20, 40].
One type of non-invasive lengthening method utilizes external electromagnetic energy to allow the release of potential energy stored within a spring inside the prosthesis, which allows for expansion (Repiphysis Limb Salvage System, Wright Medical Technology, Arlington, TN, USA; formerly known as the Phenix prosthesis) [40]. Lengthening can be performed on an outpatient basis, without anesthesia or an incision required. Generally, the prosthesis is lengthened between 6 and 10 mm per expansion (Figs. 4.6a, b, and 4.7).
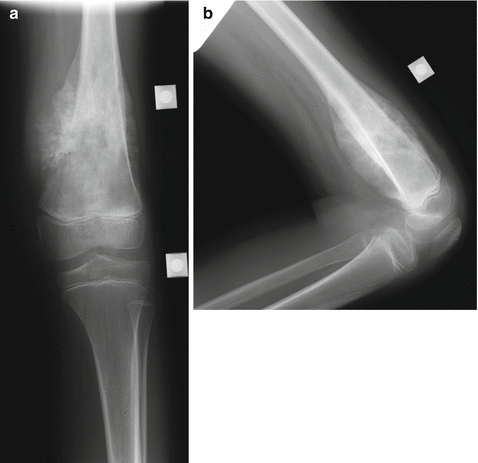
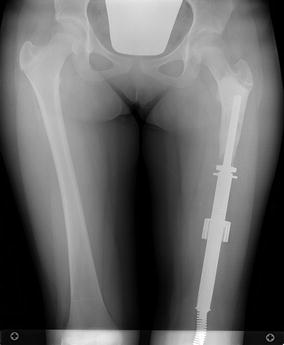

Fig. 4.6
(a, b) AP (a) and lateral (b) x-rays of the distal femur, showing Ewing’s sarcoma in this 8-year-old patient. Note the large soft tissue mass

Fig. 4.7
AP scanogram x-ray of the patient in Fig. 4.6, after resection of the distal femur and reconstruction with a Repiphysis noninvasive expandable prosthesis
Another non-invasive expandable prosthesis involves a magnet, a gearbox, and a telescoping screw (Juvenile Tumor System, Stanmore Implants Worldwide, Stanmore, United Kingdom). The magnet is activated by an external electromagnetic force, which then initiates the gearbox, which in turn drives the threaded screw to lengthen the prosthesis [20]. Like the Repiphysis, the Stanmore prosthesis can be lengthened in the outpatient setting, without any incisions or anesthesia necessary (Fig. 4.8a, b).
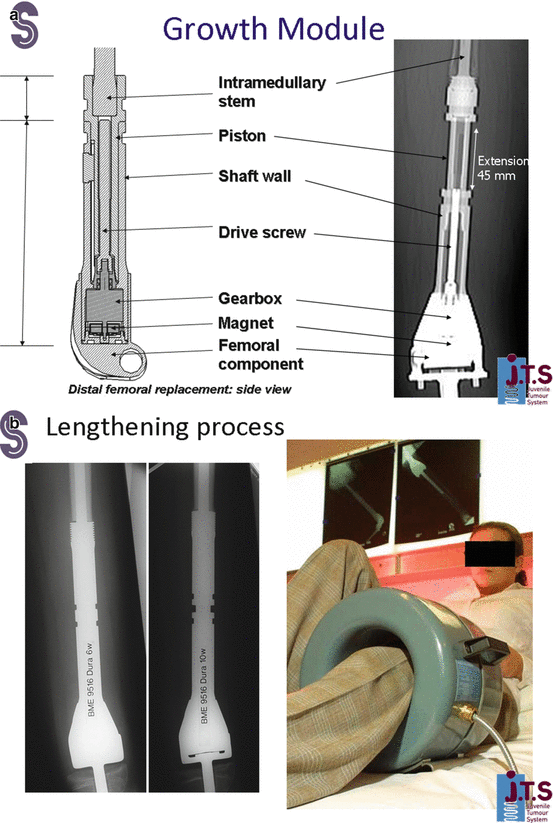

Fig. 4.8
(a, b) Stanmore method for lengthening of expandable prosthesis
Neel and colleagues reported on their experience with the Repiphysis prosthesis in 2003. Eighteen prostheses were implanted in 15 patients. Three patients in the study had to have their prosthesis revised to a new expandable prosthesis due to failure of the electromagnetic expansion portion failed. One patient underwent an above-knee amputation 10 months after surgery for an arterial thrombosis. There were eight revisions in seven patients, mostly due to either prosthesis failure or fracture. A total of 60 lengthenings have been performed, with all but two performed on an outpatient basis, and with an average of 8.5 mm of length gained at each procedure. There were no neurovascular injuries or significant loss of range of motion after any lengthening procedure. There were no deep infections. The average MSTS functional score at final follow-up in surviving patients was 90 %. All three of the patients that had achieved skeletal maturity by the time of publication had leg length discrepancies of less than 10 mm [40].
Cipriano and colleagues reported their experience with the Repiphysis prosthesis in 2014. They also noted a high complication and revision rate (12 patients had 37 implant related complications requiring 15 reoperations). Half of the patients had to undergo revision of the prosthesis for aseptic loosening. Furthermore, they noted severe peri-implant bone loss, which often limited subsequent revision options. While only one patient developed a deep infection, they saw frequent problems with arthrofibrosis, flexion contractures, extensor lags, and patellar maltracking. Contrary to Neel’s study reporting excellent MSTS scores, Cipriano’s patients only achieved a mean MSTS score of 67 % [12].
In 2006, Gupta and colleagues published their experience with the Stanmore prosthesis. In their early experience, this prosthesis was implanted into seven patients. Lengthenings were performed on an outpatient basis without anesthesia. The average length gained per procedure was 4 mm, with patients undergoing anywhere between one and 14 lengthening procedures. No neurovascular compromise was seen. One patient developed a 25° knee flexion contracture, which was treated successfully with manipulation under anesthesia and serial casting. There were no instances of deep infection, implant failure, aseptic loosening, or local tumor recurrence [20]. Their medium term results were subsequently published in 2012, incorporating 55 patients between 2002 and 2009, with mean follow-up of 41.2 months. Their complication rate was 29.1 % overall, with six deep infections (10.9 %). Three patients had a mechanical failure of the extension mechanism. Ultimately ten patients underwent a revision of their prosthesis (18.2 %). The mean MSTS score was 82.3 %, and Toronto Extremity Salvage score (TESS) was 92.3 %. Ten out of the 11 patients who had reached skeletal maturity at the time of publication had equal leg lengths, and 9 of the 11 had full hip and knee range of motion [44].
Hwang and colleagues published their experience with the Stanmore prosthesis in 2012 as well. They reported on 34 patients over 7 years, with an average follow-up of 44 months. Three patients had a local recurrence and went on to amputation. There were two mechanical failures for implant breakage, one at the sliding component and one at the lengthening mechanism. Six patients developed a deep infection (18 %). Eleven of 32 patients required subsequent surgery, with five patients going on to amputation (3 for local recurrence and 2 for infection). Despite the high complication rate, the mean MSTS score at latest follow-up was 85 % [28].
Some groups have sought to compare older invasive expandable prosthesis designs with newer non-invasive ones. Henderson and colleagues published their 13 year experience with expandable prostheses at a single institution, incorporating 39 patients. Three different implants were utilized. Early patients were treated with open expansion involving 1 cm incremental lengthening by placing a metal spacer secured by a locking clip (12 patients). As prosthesis design improved, subsequent patients had a minimally invasive expandable prosthesis placed, which could be lengthened by inserting a screwdriver through a stab incision under fluoroscopy to actuate the lengthening mechanism (18 patients). More recently, as non-invasive expandable prostheses became available, nine patients had a Stanmore Juvenile Tumor System prosthesis placed. The overall complication rate was 42 %, with 10 out of 26 surviving patients requiring revision surgery (38 %). There were three deep infections, all of which occurred after a surgical lengthening procedure; there were no deep infections in the Stanmore group, though they had the shortest follow-up time. The mean MSTS score was 87 % and the mean Pediatric Outcomes Data Collection Instrument (PODCI) score was 85.8. The authors concluded that patients undergoing expandable prosthesis placement after malignant bone tumor resection have good physical and emotional function, but that complication rates remain high. In those patients that had reached skeletal maturity, the mean leg length discrepancy was 7 mm [24].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree