Diarrhea-predominant IBS
• GI infections
• Inflammatory bowel disease
• Celiac disease
• Carbohydrate malabsorption (lactose, sucrose, fructose, sorbitol)
• Lymphocytic and collagenous colitis
• Food intolerance
Constipation-predominant IBS
• Celiac disease
• Hypothyroidism
• Anal sphincter/pelvic floor abnormality
• Tethered spinal cord
• Colon motility disorder
• Neoplastic disorders (rare in children)
Must include all of the following |
1.Abdominal discomfort (an uncomfortable sensation not described as pain) or pain associated with two or more of the following at least 25% of the time |
(a)Improved with defecation |
(b)Onset associated with change in frequency of stool |
(c)Onset associated with a change in form (appearance) of the stool |
2.No evidence of an inflammatory, anatomic metabolic, or neoplastic process that explains the subjects symptoms |
Abdominal pain is a prerequisite for the diagnosis of IBS. The pain can vary in intensity and location, but is usually restricted to the lower abdomen; it can be episodic or superimposed on a background of constant ache. It is usually relieved by the passage of stool or flatus and exacerbated by meals. Almost 50% of adult with IBS also have symptoms of dyspepsia and overlap between other pain-associated FGIDs and IBS has been reported [8]. Urinary bladder irritability and pelvic pain have also been associated with IBS-like symptoms.
Most patients with diarrhea-predominant IBS pass liquid or semiformed stool at frequent intervals. It can be accompanied with the passage of mucus but passage of blood is rare. A majority of patients will report difficulty falling asleep, rather than sleep disruption. In patients with constipation-predominant IBS, the constipation initially can be episodic but usually becomes continuous. With time symptoms become refractory to treatment with laxatives. Stool consistency can be hard and the stool may be narrow in caliber. It can be associated with the feeling of incomplete evacuation; the child can spend a long time sitting on the toilet straining unsuccessfully to have a bowel movement. This can lead to rectal mucosal prolapse and development of solitary rectal ulcer syndrome, associated with passage of blood in the stool and tenesmus [9]. Adults with dyssynergia, a disorder where the subject is unable to coordinate bearing down with pelvic floor relaxation during defecation, can have symptoms that mimic IBS [10]. Constipation associated with dyssynergia can improve with biofeedback training and this should be considered in the differential diagnosis in adolescents with constipation and lower abdominal pain. Some patients have periods of constipation alternating with diarrhea. Abdominal bloating, belching, and flatulence are also common symptoms.
Pathophysiology
The pathophysiology of IBS is likely to be multifactorial and alterations in GI sensory perception, central neuronal dysfunction, abnormal motility, stress, psychological abnormalities, and luminal factors have all been implicated. The submucosal nerve plexuses receive sensory input from the bowel lumen through the sensory receptors. The enteric nervous system communicates with the brain through neural pathways as well as by immune and endocrine systems. The pain signals are transmitted from the primary sensory afferent neurons with cell bodies in the dorsal root ganglia to the dorsal horn of the spinal cord. Spinal pathways run to the thalamus and relay messages to the limbic system and the sensory cortex. The combined functioning of the GI motor, sensory, and central nervous system activity is termed the brain gut axis. Abnormalities along the brain gut axis such as altered peripheral sensory perception, hypersensitivity of sensory neurons in the dorsal horn, and increased activation of brain regions associated with visceral pain sensation have been reported in IBS [11].
Visceral hyperalgesia (an exaggerated pain response to a sensory stimulus) has been reported in children with IBS [12, 13]. Visceral hyperalgesia could result from sensitization of primary sensory afferent fibers innervating the gut or the neurons receiving input from visceral afferents along the brain–gut axis (Fig. 46.1) [11]. Peripheral sensitization of nerves within the GI tract can result from noxious injury and the release of inflammatory mediators and nerve growth factor by the fibroblasts and mast cells in the bowel wall. The resulting increase in transcription of the neuropeptides, substance P, and calcitonin gene-related peptide initiates nerve activation and the release of yet more substance P and recruitment of previously silent nociceptors [11].
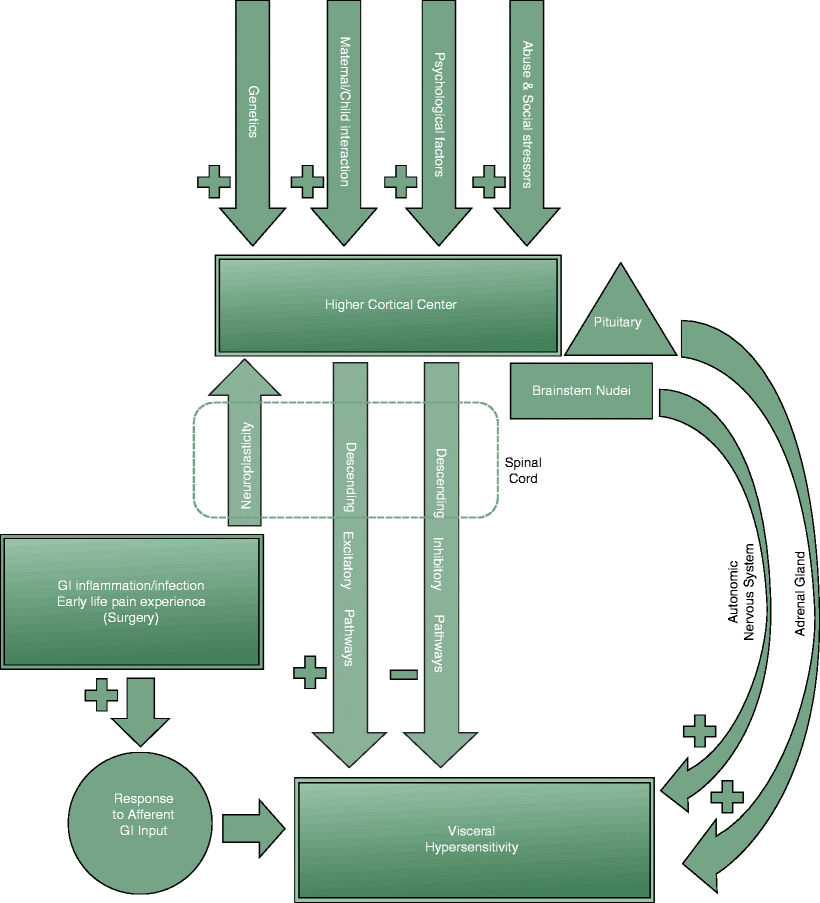

Fig. 46.1
Diagram showing the various factors contributing to the development of visceral hypersensitivity
Alteration in sensory perception in the brain centers has also been reported in adults with IBS. Tillisch et al. conducted a meta-analysis of 18 studies in which functional MRI or PET scans of the brain had been performed together with balloon distension of the rectum in patients with IBS and healthy controls [14]. Patients with IBS demonstrated a greater spatial extent of brain activity than controls, specifically in regions associated with pain modulation and emotional arousal. The authors concluded that their findings support a role for central nervous system dysregulation in patients with IBS [14].
Low-Grade Inflammation
Following gastroenteritis 7–31% of adults develop persistent low-grade inflammation and IBS-like symptoms [15–18]. A study of a large outbreak of waterborne infection with Campylobacter jejuni and Escherichia coli O157 in Walkerton, Ontario yielded 228 cases of postinfectious IBS and 581 controls who had fully recovered. This study found a number of single nucleotide polymorphisms that distinguished postinfectious IBS patients from infected controls who had fully recovered [19]. The relevant genes were CDH1 coding for E-cadherin, a tight junction protein controlling gut permeability, Toll-like receptor (TLR) that mediates the cellular response to bacterial DNA, and IL-6 [19]. TLRs are normally downregulated to avoid inappropriate activation of the immune system by gut commensals [20]. Recently increased expression of TLR-4 has been reported in females with IBS, predominately of mixed or diarrhea-predominant IBS [21]. Increased intraepithelial and lamina propria lymphocytic infiltration, together with an increase in enteroendocrine cells, have also been reported in bowel biopsies obtained from postinfectious IBS patients [18]. These changes can persist for up to 12 months and are associated with increased mucosal permeability [15, 18]. In children with IBS, immune cells presence in the rectal mucosa was associated with a higher availability of 5-HT with higher 5-HT content and lower SERT mRNA compared to control subjects suggesting that mucosal inflammation may induce peripheral sensitization [22]. Bacterial gastroenteritis and Henoch–Schonlein purpura during early childhood can lead to development of IBS-like symptoms in later life [23, 24]. Bowel inflammation and pain in early childhood may lead to alteration in afferent signal processing due to neuroplasticity which can manifest in later life with functional pain during psychosocial stress.
Gut Microbiota
Studies using fluorescent in situ hybridization to detect bacterial 16s RNA suggest that there is an increase in bacteria within the mucus layer in IBS patients [25]. Recently great advances have been made in understanding the microbiota through the development of culture-independent technologies and, in particular, metagenomics. There is great diversity and interpersonal variation in the bacterial species and strains present in the gut microbiota. Although studies of fecal microbiota in IBS are limited, a recent pediatric study reported a significantly greater percentage of the class γ-proteobacteria especially Haemophilus parainfluenzae in IBS patients. A Ruminococcus-like microbe was also more common in IBS subjects compared to controls in this study [26]. Several adult studies have reported reduced biodiversity of gut microbiota in IBS patients [27].
Altered Motility
Abnormal rectal, colon, and small bowel motility has been implicated in IBS pathophysiology. Interpretation of colon motility studies in adults with IBS is hampered by a relatively primitive understanding of normal colon motility and its intrinsic variability. Abnormalities in colon motility and abnormalities in response to food and stress have been reported in patients with IBS [28]. Abnormalities in small bowel motility such as repetitive bursts of contractions or clusters, prominent high amplitude waves in the terminal ileum, and an exaggerated jejunal motor response to a meal have also been reported in adults with IBS [28, 29].
There is also a suggestion that IBS patients handle small bowel gas differently and there is slow transit of gas directly infused into the small bowel in adults with IBS [28]. Abdominal bloating and flatulence can also result from higher colonic fermentation in IBS [28, 30, 31]. Some patients without evidence of small bowel bacterial overgrowth can benefit from treatment with unabsorbable antibiotics [32], which raises the question of a qualitative change in bowel bacterial flora in IBS.
Biochemical Changes
Serotonin (5-hydroxytryptamine: 5HT) is secreted in copious amounts by the gut enteroendocrine cells and serves as a critical messenger for GI fluid secretion and motility. It activates at least five different receptor types, and the 5-HT3 and 5-HT4 receptors are the most extensively studied in IBS [33]. The transporter of 5-HT (SERT) mediates the reuptake of 5-HT by the neurons and crypt epithelial cells and terminates its action.
Plasma 5-HT concentration is elevated in IBS patients [34] and the proportion of 5-HT secreting enteroendocrine cells is elevated in the GI tract in postinfectious IBS patients [15]. Increased rectal mucosal 5-HT concentration has also been reported in children with IBS. Presence of low-grade inflammation was associated with higher 5-HT concentration in rectal mucosa in this study [22]. Symptom relief by serotonergic agents including 5HT3 antagonists and 5HT4 agonists provides additional support for a possible role of 5-HT in IBS pathophysiology [35].
Genetics
Familial aggregation and twin studies suggest that there may be a genetic predisposition to developing IBS [36, 37]. Twin studies have shown that the concordance rate for IBS is higher in monozygotic compared to dizygotic twins [38]. However, presence of IBS in the respondent’s parents made a much larger contribution to the risk of having IBS than did the presence of IBS in one’s twin, suggesting social learning may be more important than the environmental factors in determining illness behavior [38]. Family members of IBS patients are more likely to have the condition, compared to their spouse controls. To date, nearly 60 genes involved in different pathways including serotonin, adrenergic, inflammation, and intestinal barrier function have been studied to determine whether specific genetic variants may be associated with IBS [39]. Interleukin 10 is an anti-inflammatory cytokine and fewer IBS patients have the high IL-10 producing (G/G) genotype compared to healthy controls [36]. Four different studies have explored the association of SERT gene polymorphism in IBS [36]. SERT is important for terminating the GI activity of 5-HT. The wild type l/l polymorphism results in normal function, whereas the presence of the short allele (s/l or s/s) results in impaired SERT function. As a group, SERT polymorphism was similar in healthy subjects and IBS patients, but some differences were observed in subgroups of IBS patients, and these differences could be population specific.
Psychological Factors
Community-based studies in adults have shown that IBS patients are indistinguishable from the rest of the population in terms of psychological comorbidities [40]. Higher psychological comorbidities have been reported in a subset of IBS patients who seek medical help [40]. Patients with psychosomatic disorders such as depression have activation of the immune system and elevated CRP [41]. Adults who develop postinfectious IBS are more likely to develop depression [42] and depressive symptoms have also been linked to relapses of colitis [43] and disease activity [44] in IBD patients. It is not clear if the depression is the result of chronic ill health or leads to the development of IBS. In children social learning of illness behavior can also contribute to the development of IBS; children of mothers with IBS are more likely to seek medical help for functional gastrointestinal symptoms [45]. Children with IBS who have significant psychological comorbidities run a more protracted illness course and are less likely to respond to treatment [46].
Functional Pain and IBD
Bowel injury and inflammation can induce functional and structural changes in the enteric neurons and muscles. Increased numbers of ganglion cells, axonal degeneration, and a reduced number of interstitial cells of Cajal have been reported in IBD [18]. In Crohn disease there is increase in substance P and its receptors in the GI tract [18]. The bowel innervation shifts from a predominantly cholinergic to a substance P predominant innervation in ulcerative colitis (UC) [18]. Increased number of nerve fibers expressing transient receptor potential vanilloid type 1 (TRPV1) receptor has been reported in the gastrointestinal tract of patients with IBD and IBS [47]. Subjects with quiescent IBD who have IBS like symptoms also have increased TRPV1 receptor expression [48]. The TRPVI expression on afferent pain fibers is upregulated by inflammation [48]. These changes can cause alteration in bowel sensory perception. Patients with active UC show a decreased threshold for painful and nonpainful rectal distension stimulus [49]. The hypersensitivity can be widespread and a lower pain threshold to esophageal distension has been reported in adults with UC [50]. In contrast, patients with isolated ileal Crohn disease have an increased pain threshold following rectal distension [51]. It appears that the development of visceral hypersensitivity in IBD may depend on the disease activity, type of inflammation, and region of the GI tract involved.
There is a considerable overlap between IBS and IBD symptoms. Adults who develop IBD may have a prodrome of IBS-like symptoms that can be as long as 7 years [52]. Some of these patients could have a delayed diagnosis of IBD, but some may have GI inflammation not severe enough to make a diagnosis of IBD, but sufficient to cause IBS-like symptoms. Up to 57% of adults with Crohn disease and 33% with UC have symptoms like pain and bloating when in clinical, laboratory, and endoscopic disease remission [53]. Since a few inflammatory cells located strategically near the enteric nerves or myenteric ganglion cells can alter bowel function in IBS, similar changes could be responsible for the functional symptoms in Crohn disease patients, which causes transmural inflammation [54–56].
Evaluation of placebo response in Crohn disease provides indirect evidence to the existence of functional GI disorders in these patients. Placebo therapy can alter the natural course of Crohn disease. In a meta-analysis of 23 adult studies using Crohn Disease Activity Index (CDAI) to measure Crohn disease activity, the pooled median remission rate with placebo was 19% (range 0–50%) [57]. Significant predictors of a placebo response were duration of participation in the study and number of clinic visits. The placebo effect increased with the increasing study duration (Fig. 46.2), suggesting that frequent contact with medical professionals relieved symptoms in some patients. A high CDAI and CRP at recruitment showed a negative correlation with the placebo response, suggesting that patients with a low or normal CRP and a comparatively mild clinical disease activity were more likely to respond to a placebo. Therefore the obvious question is whether some of these patients with Crohn disease had functional GI symptoms to begin with and were therefore more likely to respond to a placebo.
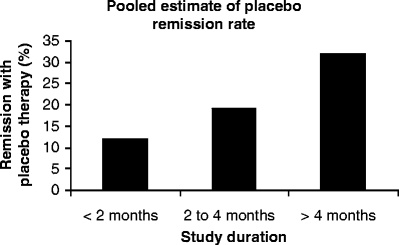

Fig. 46.2
Data from meta-analysis of 23 studies that used CDAI to measure disease activity in Crohn disease. The pooled estimate of remission with placebo therapy increased with study duration
Diagnosis
The diagnosis of IBS is based on clinical symptoms and signs (Fig. 46.3). Investigative work up and endoscopic evaluation may be necessary in a small percentage of children especially in the presence of alarm features. In one large pediatric study, anemia, hematochezia, and weight loss were most predictive of Crohn disease in children presenting with chronic abdominal pain, with a cumulative sensitivity of 94% and specificity of 62% [7]. Adult studies suggest that 5–17% of celiac disease patients have IBS-like symptoms and in one study of 1,032 adults with celiac disease 37% were diagnosed with IBS prior to the diagnosis of celiac disease [58]. Abdominal pain is a common symptom in children with celiac disease but the prevalence of IBS is unknown. More than 90% of these adults have improvement in IBS-like symptoms after starting gluten-free diet. Lactose intolerance has been reported in 15–25% of adults with IBS. However, it is yet to be determined if lactose exclusion results in resolution of IBS symptoms.
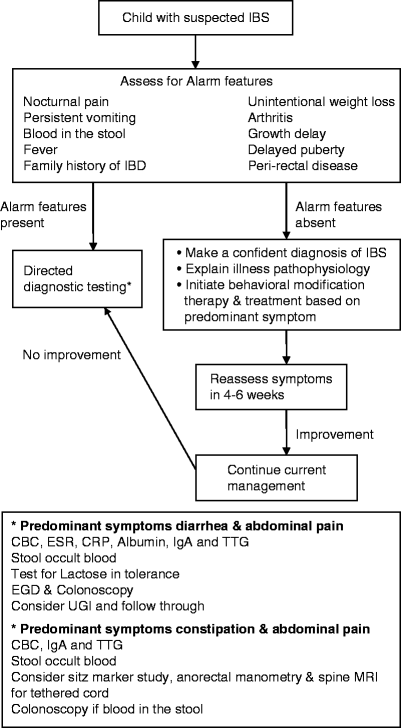

Fig. 46.3
Algorithm for management of children presenting with symptoms consistent with the diagnosis of IBS
Treatment
When evaluating children with IBS it is important to allocate sufficient time for the consult to allow the child and family to share their concerns. One must acknowledge the presence of pain, adopt an empathic and nonjudgmental point of view, educate and reassure the child and the parents by explaining the source of symptoms in the absence of an identifiable cause [59]. It should also be made clear that the improvement will be slow and the focus should be on normalization of psychosocial functioning, rather than trying to identify the cause for the symptoms.
Cognitive behavioral therapy (CBT), family intervention and guided imagery, a form of relaxed and focused concentration, have been successfully used to treat functional abdominal pain in children and are also effective in IBS [59]. Adult studies have shown that attention management techniques such as hypnosis and mindfulness meditation are useful to treat IBS symptoms. Patients with prolonged illness and complex psychological comorbidities, which interfere with participation in a treatment plan, may require early referral to a multidisciplinary team, which includes a pain psychologist and a gastroenterologist [46]. CBT is based on the belief that our thoughts, behaviors, and feelings interact, and CBT aims to reduce or eliminate physical symptoms through cognitive and behavioral changes. It guides the patient to modify or change cognitive distortions and negative thinking and enables the patient to substitute these with more realistic thoughts, such as that the pain is likely to subside and does not represent a terminal illness. Several randomized controlled trials to test the effectiveness of pain interventions in children with functional abdominal pain using a self-management approach that includes component of CBT have yielded encouraging results. However, methodological difficulties and different criteria used to classify patients can make interpretation difficult. Cognitive behavioral and relaxation therapy are emerging as the first-line treatment for children with functional abdominal pain and are also useful to treat children with IBS.
Dietary triggers such as caffeine, fatty meals, and carbonated soft drinks should be eliminated. A lactose free diet can help patients with IBS symptoms associated with lactose intolerance. Increasing dietary intake of fiber can help patients with constipation-predominant IBS, but metabolism of the bulking agents by gut bacteria can produce gas, which can worsen symptoms of bloating and flatulence. A meta-analysis in adults with IBS suggested that soluble fiber sources such as psyllium, ispaghula, and calcium polycarbophil may be more effective in improving global IBS symptoms compared to insoluble fiber [60, 61]. An innovative approach for treatment of IBS in adults comprises a reduction in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) in the diet [62, 63]. These short chain carbohydrates have common functional properties in that they are poorly absorbed, osmotically active, and rapidly fermented by bacteria. A randomized placebo controlled trial in adults demonstrated that, in patients with IBS whose symptoms improved on a low FODMAP diet, recurrence of symptoms occurred on rechallenge with fructose, fructans, and a combination of the two, but not placebo [62].
Polyethylene glycol 3350 and milk of magnesia can be used as a stool softener in patients with constipation. Lubiprostone, a type 2 chloride channel agonist, is effective in treating constipation and constipation-predominant IBS in adults [64]. Pediatric trials documenting efficacy in pediatric age group are lacking. Menthol, the active ingredient in peppermint, inhibits smooth muscle contractions by blocking calcium channels. Enteric-coated peppermint oil capsules can help relieve abdominal pain [65]. Peppermint oil can however cause rectal burning, esophageal pain, and allergic reactions [65]. There are no controlled studies in children showing the efficacy of anticholinergics in IBS and adult trials have also produced conflicting results. In general, the anticholinergic effect in adults with IBS is comparable to a placebo [65]. The authors do not prescribe antispasmodics, but if a patient is already using them and finds them useful then the authors do not discontinue the medication. Loperamide can be useful to reduce the stool frequency in diarrhea-predominant IBS patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


