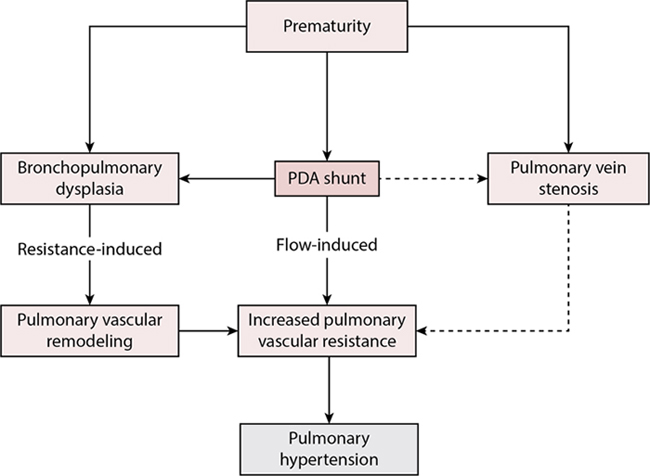
Adrianne Rahde Bischoff, Carl Backes, Dany E. Weisz, Bassel Mohammad Nijres, Patrick J. McNamara Key points The decision for definitive closure of a patent ductus arteriosus (PDA) remains one of the biggest controversies in neonatology. Importantly, there is marked center-to-center variation in the incidence of ligation among extremely-low-birth-weight (ELBW) infants, and overall rates of surgical ligation reported by international neonatal networks are decreasing.1,2 The reasons behind this secular trend are likely multifactorial, but in many centers surgical ligation is being substituted, and even superseded, by the practice of percutaneous closure.3 Percutaneous (or transcatheter) PDA closure is an emerging technique that has gained popularity in the most recent years and is being performed in progressively smaller and younger patients. Advancements in the technique with decreased fluoroscopy time, venous retrograde approach, the launch of devices that are more appropriate for the preterm ductal morphology, and increased experience have expanded the use of percutaneous closure, with high rates of technical success and few major adverse events.4 Observational studies have associated PDA surgery with adverse neonatal outcomes and neurodevelopmental impairment (NDI) in early childhood, although bias due to residual confounding threatens the validity of these studies. Infants treated with surgical ligation undergo major rapid changes in systemic hemodynamics, which commonly lead to postoperative cardiorespiratory instability. In addition, given that dependence on mechanical ventilation is the sine qua non of referring an infant for definitive PDA closure, the increasing availability and use of advanced noninvasive methods of ventilation may permit earlier endotracheal extubation of extremely preterm infants. Consequently, clinician perceptions of the merits of a definitive intervention may differ, prompting them to avoid interventional PDA closure, with the hope of spontaneous ductal closure. It’s important to note though that, specifically in ELBW, the rate of spontaneous closure is poor; more than 50% of infants born <750 g still have a PDA around day 50.5 Additionally, the impact of chronic pulmonary overcirculation on the pulmonary parenchyma and on pulmonary vasculature remodeling must be appreciated. Despite several controversies in the literature regarding the impact of PDA closure on different neonatal outcomes, there is increasing evidence of a correlation between prolonged shunt exposure and the development of bronchopulmonary dysplasia (BPD). Although the TIPP trial showed no decrease in the incidence of BPD with the use of prophylactic indomethacin,6 one recent study reported that a change toward a strict nonintervention approach to PDA resulted in an absolute increase of 31% in BPD in infants born less than 26 weeks.7 Prolonged exposure to shunts also has a detrimental impact on pulmonary vascular development, and the presence of an untreated large PDA in the first 2 years of life may result in pulmonary vascular disease in as many as 50% of patients.8,9 Philip et al. demonstrated that exposure to a PDA beyond 8 weeks of life is associated with an elevation in pulmonary vascular resistance (PVR) index, greater respiratory severity score, and prolonged use of mechanical ventilation.10 In preterm infants, who have a smaller cross-sectional area of pulmonary vessels and several other inflammatory risk factors, the elevation in PVR occurs more frequently and at an earlier age,9,11,12 while prolonged PDA exposure is also a potential contributor in the development of pulmonary vein stenosis. Figure 18.1 summarizes the multiple contributors to the pathophysiology of PDA shunt-related pulmonary hypertension. Although the risks of prolonged shunts are relatively well established, the timing and indication for definitive closure have not been rigorously evaluated. Simultaneously, although the percutaneous approach is deemed to be less invasive and with less adverse events, the evidence regarding appropriate timing and indication for referral remain unknown. As a result, contemporary practice is dominated by considerable uncertainty regarding the role of definitive closure. In this chapter we review the evidence regarding the benefits and risks of PDA interventional closure in very preterm neonates and provide a pathophysiology-based management paradigm to guide peri-closure care in these high-risk infants. In contemporary practice the “definitive PDA closure decision” remains an enduring controversy for clinicians. There is currently a paucity of knowledge regarding the clinical and echocardiography characteristics of infants with persistent PDA who may benefit from definitive closure. The relative risks and benefits of interventional closure compared with conservative management are unknown. Although dependence on mechanical ventilation remains the primary criterion for definitive closure referral, the relative independent contribution of the PDA to ongoing respiratory insufficiency is, at present, difficult to quantify. Infants with similar echocardiography indices of PDA hemodynamic significance may have varying degrees of respiratory failure (none to severe) owing to differences in nascent lung disease of prematurity, pulmonary arterial pressure, and tolerance of the increased pulmonary blood flow from the ductal shunt. Limited evidence from clinical trials and careful reflection on contemporary treatment practices provides some boundaries for surgical treatment, mostly by limiting the use of ligation in the first postnatal week. Although the trial by Cassady et al. reported reduced necrotizing enterocolitis (NEC) rates with prophylactic surgical ligation on the first postnatal day in ELBW infants, several factors render this practice now untenable.13 First, prophylactic indomethacin has both relatively high efficacy for early closure and reduces all grades of intraventricular hemorrhage (IVH), providing a therapeutic alternative with additional benefit. Second, early PDA ligation may result in increased right ventricular (RV) afterload, so early ligation may be harmful in preterm infants with increased pulmonary artery pressure, a common finding in severe respiratory distress syndrome. Finally, the natural history of ductal closure has been described, with many infants experiencing early spontaneous closure. Exposing all infants to the risks of interventional closure is therefore inappropriate.14 Persistent ductal shunt may be considered a chronic, consistent contributor to impaired pulmonary compliance. Infants with a persistent PDA may be considered for interventional closure when the clinical and echocardiography evaluations identify a shunt of sufficient volume that may be actively contributing to respiratory insufficiency. Echocardiography markers of ductal significance may include measurements of left heart volume loading (i.e., pulmonary vein D wave velocity, mitral E wave velocity, left atrium to aorta ratio and isovolumic relaxation time), estimates of Qp:Qs (left-to-right ventricular output ratio) and of systemic hypoperfusion (diastolic flow reversal in the descending aorta and/or systemic vessels such as celiac and middle cerebral artery).15,16 Persistent dependence on invasive or noninvasive ventilation in combination with moderate-severe echocardiography indicators of hemodynamic significance suggests an impaired ability to compensate for the ductal shunt. It is, however, important to consider preterm infants who have clinical features of chronic end-organ hypoperfusion (systemic hypotension, renal failure, feeding intolerance) in the absence of an acute etiology (e.g., sepsis, NEC) accompanied by echocardiographic indices of a large ductal shunt. In these infants echocardiography indices are often in the “severe” range across all parameters, although there are little data regarding the direct clinical relevance of these deviations from normal. Early interventional closure after failure of medical therapy may be indicated for this subgroup of infants as well as a strategy to prevent BPD and pulmonary vascular remodeling in the most immature high-risk patients. The clinical decision to treat a preterm neonate with surgical PDA ligation is controversial, owing to uncertainty regarding the impact of PDA surgery on neonatal and neurodevelopmental outcomes. A limited number of randomized clinical trials, all conducted more than 3 decades ago, have evaluated the impact of surgical ligation in preterm infants on neonatal outcomes.17–19 A common salient feature of these studies is a lack of external validity to permit their interpretation within modern neonatal intensive care; these trials were either performed prior to the availability of pharmacological PDA treatment, identified “hemodynamically significant” PDA based on clinical exam rather than echocardiography, and/or enrolled relatively mature preterm neonates, and thus bear minimal resemblance to care provided to the micropremature neonates being considered for PDA surgery in contemporary practice. In lieu, observational studies have contributed most prominently to the evidence of the potential effect of PDA ligation on clinical outcomes. Large retrospective cohort studies have associated PDA ligation with increased neonatal morbidity and NDI in early childhood.20–25 A meta-analysis of randomized trials and adjusted observational studies demonstrated that, compared with medically treated infants, ligated infants were more likely to develop moderate-severe BPD, severe retinopathy of prematurity, and moderate-severe NDI, although with improved survival.26 In light of concerns regarding NDI and neonatal morbidities, the safety of PDA ligation has been questioned.27 These concerns have been associated with a secular trend toward a reduction in infants being treated with surgical ligation in North American centers.1,2 However, residual confounding bias threatens the validity of observational studies that have associated PDA ligation with increased neonatal morbidity and NDI compared with medical management alone. Most studies only adjusted for antenatal and perinatal confounders (e.g., gestational age). Because ligation typically occurs several weeks after birth, such studies likely inadequately addressed confounding by indication – those infants referred for ligation may have been more “ill” and/or have larger ductal shunts at the time of the decision to treat with surgery, compared with infants who are treated with medical management alone. Illness severity, characterized by postnatal pre-ligation morbidities such as intraventricular hemorrhage and sepsis, and intensive care parameters such as dependence on mechanical ventilation, are important confounders as they are associated with both the decision to treat with PDA ligation and also with neonatal morbidity and NDI. The potential effect of residual confounding bias in past studies was highlighted in a recent large multicenter cohort study of extremely preterm neonates with PDA, where after adjustment for postnatal pre-ligation confounders, ligation was no longer associated with adverse outcomes.28 These findings have direct clinical relevance; specifically, neonatologists and pediatric cardiac surgeons may now consider to no longer prioritize the risk of adverse neonatal and neurodevelopmental outcomes as a reason to avoid surgical PDA ligation. Traditionally, technical success in percutaneous PDA closure is defined as placement of the device in the catheterization laboratory. Alternatively, technical failure is defined as the inability to place a device (or device placement, but subsequent need for removal due to malposition).29 In a meta-analysis that synthesized studies (1994–2016), 635 infants <6 kg undergoing percutaneous PDA closure were identified.30 Among that cohort, the authors observed a technical success rate of 92% (95% confidence interval [CI] 88.8–95.0]; rates of any complications and clinically significant complications were 23.3% (95% CI 6.5–30.8) and 10.1% (95% CI 7.8–12.5), respectively.30 Using similar definitions, a more contemporary meta-analysis (2021) from Bischoff et al. investigated the technical success and safety profile of percutaneous closure among 373 infants <1.5 kg.4 Interestingly, the authors observed procedural success rates of 96% (95% CI 93–98%), while rates of any complications and clinically significant complications were 27% (95% CI 17–38%) and 8% (95% CI 5–10%), respectively.4 To further increase rates of technical success and minimize adverse events, a leadership panel of pediatric interventional cardiologists recently published consensus-based guidelines that address pre-, intra-, and post-procedural considerations of percutaneous PDA closure in lower-weight infants.31 Thus risk-benefit profiles for the procedure are expected to continue to improve over the next decade.32 However, in the absence of direct comparison of percutaneous closure versus alternative treatments, available risk/benefit profiles for the procedure lack the requisite context to guide evidence-based clinical practice. Despite high rates of technical success with “off-label” use of various devices,4,30,33 the need for percutaneous PDA closure devices that address the unique ductal morphology and profile of preterm infants was clear.30,34–38 Accordingly, over the past decade, in the spirit of collaboration and innovation, the pediatric cardiology community and private industry have partnered to design PDA closure devices specifically tailored for the preterm infant.39,40 For example, the Amplatzer Piccolo Occluder was modified (short-length, low-profile delivery system) to make it suitable for use in preterm infants.41 In fact, following successful execution of a multicenter, non-randomized clinical trial, the Amplatzer Piccolo Occluder (or Amplatzer Duct Occluder II Additional Sizes, ADO-II AS; Abbott, Chicago, Illinois) was approved (2019) by the Food and Drug Administration (FDA) for percutaneous PDA closure in preterm infants >3 postnatal days and weighing >700 g.41,42 Of note, the Amplatzer Piccolo Occluder is FDA approved for PDAs ≤4 mm in diameter; thus off-label use of alternative devices remains common.39 For example, leading investigators recently reported that the Microvascular Plug 7Q is safe and feasible among premature infants with PDAs >4 mm in diameter.43 Although the ductus in preterm infants is typically long and tubular, marked variability in ductal length demands that a variety of device modalities be available.32,44 In the absence of comparative studies the optimal device to close the PDA remains at the discretion of the interventional cardiologist and various institution and individual providers.33 Surgical PDA ligation via thoracotomy was the traditional method of procedural PDA closure.45 However, over the last 2 decades, associations between surgical ligation and adverse outcomes, including neurodevelopmental delays, have emerged.46,47 These observations have led to growing interest among the pediatric health care community in percutaneous PDA closure as a treatment to achieve definitive ductal closure. A recent study using the Pediatric Health Information System (PHIS) database compared short-term outcomes among infants undergoing definitive ductal closure, including percutaneous (n = 175) or surgical PDA closure. Although infants undergoing percutaneous ductal closure were older (0.53 vs. 0.1 years, P < 0.001) and less premature (20% vs. 60%; P <0.001), the authors observed that those undergoing percutaneous closure had lower mortality (0% vs. 1.7%, P = 0.02) and reduced hospital length of stay (difference in 3 days; 95% CI 1.1–4.9 days; P = 0.002) than those undergoing surgical ligation.48 Other investigators have observed similar rates of safety and feasibility between percutaneous PDA closure versus surgical ligation.49 In the absence of contemporary randomized controlled trials (RCTs) comparing percutaneous closure versus surgical ligation, fundamental questions on the best approach to achieve definitive ductal closure will remain unanswered.33 In view of the lack of comparative data, a multidisciplinary leadership panel convened and developed research priorities for the use of percutaneous PDA closure.50,51 The need for a multicenter RCT of percutaneous PDA closure, with well-defined inclusion/exclusion criteria, rigorously applied treatment protocols, consideration of potentially harmful exposures (e.g., cardiac anesthesia), and longer-term neurodevelopmental assessments, was emphasized.51 Consistent with US trends in surgical PDA ligation rates,45 the panel did not have interest or equipoise for a comparison of percutaneous closure versus surgical ligation. In fact, in view of widespread acceptance in the health care community, conservative management (“watchful waiting,” diuretics, fluid restriction) was determined to be the best comparative treatment modality.52 Following iterative discussions, an National Institute of Health (NIH)–funded study comparing percutaneous PDA closure versus conservative treatment (“PIVOTAL: Percutaneous Intervention Versus Observational Trial of Arterial Ductus in Lower Gestational Age Infants”) is planned to begin recruitment in 2023 (NCT #03982342).53 Pre-closure evaluation is aimed at verifying ongoing suitability for PDA closure, optimizing stability to improve procedural tolerance, and identifying neonates at modifiable risk of post-closure morbidity. Repeat echocardiography should be performed within 48 hours of definitive closure to confirm PDA severity and rule out the development of spontaneous ductal closure or constriction, which occurs in a small but important minority of extremely preterm neonates after referral for ligation.54 Baseline hemoglobin, platelet count, and prothrombin time should be obtained for correction of anemia and verification of normal coagulation function. Adrenocorticotropin stimulation testing can be performed preoperatively to assess adrenal cortex responsiveness, which may be impaired in preterm infants with PDA.55 Preoperative serum cortisol ≤750 nmol/L (≤17 μg/dL) after adrenocorticotropic hormone (ACTH) stimulation is associated with increased postoperative hypotension and respiratory failure.51 On the other hand, routine preoperative stress-dose hydrocortisone has not been associated with improved cardiovascular stability, regardless of gestational age.56 Maintenance of euvolemia pre-closure is recommended, given that aggressive fluid restriction and use of diuretics may further exacerbate decreased systemic blood flow to post-ductal organs and have no impact on shunt volume.57 On the contrary, additional volume loading does not prevent post-closure cardiovascular instability.58 Lastly, during the procedure (ligation or percutaneous closure), neonates typically receive mechanical ventilatory support. Use of a volume-targeted, pressure-limited mode (when on conventional ventilation) may reduce the risk of baro- and volutrauma resulting from rapid changes in pulmonary compliance after interruption of the PDA shunt.59 While there are no specific respiratory strategies recommended, high-frequency modes have equivalent success to conventional mechanical ventilation in experienced centers.60 Detailed technical information about percutaneous PDA closure can be found in the Appendix 18A. PDA closure results in significant changes in loading conditions to the left ventricle (LV). Specifically, there is an instantaneous drop in LV and left atrium preload due to decreased pulmonary blood flow return, with a proportional reduction in LV output.61–64 Given that most patients are above the critical filling volume and go from a state of “high” to “normal” preload, the impact on LV contractility dictated by the Frank-Starling law is limited. In addition, the normalization of pulmonary blood flow leads to improved lung compliance and function.59,65 With the removal of the low resistance circuit of the pulmonary vasculature, the LV is exposed exclusively to systemic vascular resistance (SVR), with resultant increase in LV afterload.63 Using noninvasive electrical cardiometry to monitor hemodynamic changes intraoperatively, Lien et al. demonstrated that there was a significant decline in LV output and surge in SVR upon sudden termination of ductal shunting.66 The deterioration in LV output was associated with a decrease in stroke volume rather than heart rate, and the magnitude of the reduction was particularly pronounced among ELBW infants.66,67 The changes in loading conditions were also demonstrated after percutaneous PDA closure, with a decrease in end-diastolic and end-systolic volume, an increase in arterial and end-systolic elastance, and a decrease in LV output subsequent to the procedure.68 PDA ligation in premature baboons resulted in impaired LV systolic performance and ventilation failure, coinciding with an increase in SVR.69 LV fractional shortening (FS) deteriorates 6–12 hours after surgical ligation,69 whereas in humans there is an immediate decrease in LV FS as well as mean velocity of circumferential fiber shortening (mVCFc) that persists after 24 hours.64 Similar findings have been confirmed with modern echocardiographic analysis in preterm infants, mostly in the past 15 years.61,70 The LV myocardial performance index, which incorporates the isovolumetric contraction and relaxation times and adjusts, at least in theory, for preload by accounting for the ejection time, deteriorated acutely after ligation, mimicking the changes in LV output.63 LV efficiency, defined as the ratio of stroke volume to pressure-volume area, transiently deteriorates within 1–24 hours after ligation and then recovers to preoperative levels by 2–4 days after ligation.68,70 In a cohort of 19 very preterm infants evaluated with tissue Doppler imaging and myocardial deformation techniques after PDA ligation, systolic and diastolic LV tissue Doppler velocities and global LV longitudinal strain decreased immediately after ligation.71 The former remained lower than the preoperative levels at 18 hours, whereas the latter improved 18 hours after the procedure.71 These findings were replicated in a cohort of extremely preterm infants undergoing percutaneous PDA closure, where targeted neonatal echocardiography 1 hour after closure showed a significant decrease in global longitudinal strain, as well as myocardial work index and work efficiency.68 Notably, these longitudinal changes can be affected, to a certain extent, by the acute changes in loading conditions rather than solely myocardial performance alone.72 Saida et al. demonstrated that a larger preoperative LV internal dimension in diastole was predictive of a decrease in postoperative FS of the LV.73 Traditional echocardiography markers of LV systolic performance, such as ejection fraction, are load dependent and cannot be interpreted as an isolated index of contractility. However, there is evidence to suggest that PDA closure leads to an inverse stress-velocity relationship: a preload-independent afterload-adjusted method of assessing LV contractility.64 Preterm infants have decreased sarcoplasmic reticulum and a poorly developed or absent T-tubule system in their myocytes; thus they have less mature excitation-contraction and relaxation mechanisms.74 As a result, the ability of the myocardium of premature neonates to respond to a sudden surge in SVR is limited compared with that of term neonates.75 The risk of impaired LV systolic performance and associated clinical deterioration appears to be greater in more immature infants after PDA closure.64,75 Doppler evaluation of systemic arteries, such as the celiac, superior mesenteric, renal, and cerebral arteries, demonstrates significant postoperative changes after surgical ligation.73 Hoodbhoy et al. reported an increase in average velocities in both the celiac and superior mesenteric arteries within 3 hours post-PDA ligation, but such a phenomenon was not found in the middle cerebral artery until 24 hours after surgery.76 The normalization of Doppler estimates of diastolic flow after PDA ligation strongly implicates the PDA as the cause of abnormal preoperative diastolic flow in systemic arteries. However, the utility of these sonographic changes in managing post-closure care is unknown. The reported association of PDA ligation and neurodevelopmental impairment has led to concerns regarding the effect of postoperative myocardial dysfunction and systemic hypotension on cerebral perfusion, especially in extremely preterm infants with reduced capacity for cerebrovascular autoregulation.77 Leslie et al. evaluated cerebral electrical activity changes after ligation using amplitude-integrated electroencephalography and found a decrease in the lower trace margin and proportion of patients with trace continuity after surgery, independent of cardiac output status.78 However, other studies that have used near-infrared spectroscopy to assess changes in regional cerebral oxygen saturation and cerebral blood flow have shown conflicting results.77 There are no studies to date evaluating the effect of percutaneous PDA closure on cerebral perfusion and/or electrical activity. The relationship of post-closure brain perfusion and later neurosensory impairment requires further evaluation. The post-closure course may be complicated by acute cardiorespiratory instability. Traditional post-ligation cardiac syndrome (PLCS) occurs in up to half of infants undergoing surgical PDA ligation and typically occurs between 6 and 12 hours after surgery.63,72,79,80 The true incidence of cardiorespiratory instability has not been described after percutaneous closure,81 although in one study of patients who received targeted milrinone prophylaxis, approximately 50% of the infants developed predominant respiratory instability which unlike PDA surgery was most commonly seen in hypertensive patients.68 The two components of instability included are ventilation and/or oxygenation impairment with or without cardiovascular compromise, which may include systolic hypotension and/or need to start/increase inotropic support.63,79,82–84 Data suggests that increased LV afterload is a greater contributor to the pathophysiology of post-closure instability rather than reduced preload. Impairment in indices of LV systolic function and peak measures of LV afterload coincide with the clinical onset of instability at approximately 8 hours post-operatively.64 In contrast, the effects of reduced LV preload, such as low LV output, are echocardiographically evident as early as 1 hour after surgery when the patient is clinically asymptomatic.68,85 Reduced preload may limit the immature myocardium’s ability to maintain cardiac output under conditions of increased afterload and may be clinically important in select infants with coexisting severe acute inflammatory lesions (e.g., NEC) where capillary leak may reduce intravascular volume. The risk for vasopressor-inotrope use after surgical PDA ligation relates to lower birth weight, earlier gestational age, or higher ventilatory support.72 Retrospective studies have failed to identify any relationship between surgical technique, anesthetic approach, or intraoperative fluid management and the development of hypotension.58,84 Jain et al. reported that an LV output of less than 200 mL/kg/min estimated by echocardiography 1 hour after PDA ligation was a sensitive predictor of systolic hypotension and the need for inotropes.79 Reduced tissue Doppler systolic indices (LV basal lateral peak systolic annular velocity [S′], basal septal S′ and basal RV S′) at 1 hour postoperatively correlate strongly with early low LV output, potentially providing an additional echocardiography indicator of infants at higher risk for cardiovascular instability.85 Milrinone, a selective phosphodiesterase III inhibitor, is a systemic vasodilator with lusitropic and positive inotropic effects (see Chapter 5, Neonatal Hemodynamic Pharmacology)80 that can be used as a prophylactic agent in a selective population. Jain et al. demonstrated that early postoperative administration of milrinone in infants with low cardiac output (<200 mL/kg/min) was associated with a significant reduction in ventilation failure (from 48% to 15%), need for inotropes (from 56% to 19%), and the incidence of overall PLCS (from 44% to 11%).79 Milrinone treatment was also associated with longitudinal improvement in tissue Doppler imaging and speckle-tracking echocardiography–derived indicators of systolic function within 18 hours, likely due to the effects of afterload reduction and improvement in myocardial systolic performance.85 This practice is supported by a randomized placebo-controlled trial of universal prophylactic milrinone after open congenital heart disease surgery in a pediatric population, which demonstrated a 64% relative risk reduction in death or low cardiac output syndrome in the first 36 hours postoperatively in the milrinone group (26.7% vs. 9.6%, P = 0.007).86 Post-closure milrinone treatment has not been evaluated in a randomized trial in preterm infants after interventional PDA closure. Postoperatively, ligation of the PDA has been shown to improve pulmonary dynamic compliance, tidal volume, and minute ventilation.65 However, not all patients exhibit immediate improvement; specifically, a subset develop early (4–12 hours after surgery) oxygenation or ventilation failure, requiring an escalation of respiratory support.64,79 In preterm baboons PDA ligation was found to produce a significant increase in the expression of genes involved in pulmonary inflammation (COX-2, tumor necrosis factor [TNF]-α, and CD14) and a significant decrease in α-epithelial sodium channel (ENaC) expression, resulting in a decrease in the rate of alveolar fluid clearance.87 This deleterious inflammatory response to the surgical procedure may, at least in part, mediate deterioration in lung function in preterm infants.88 Ting et al. reported that the incidence of oxygenation or ventilation failure remained high (51.2%) in preterm infants undergoing PDA ligation, despite the implementation of an early targeted milrinone prophylaxis approach for infants with early echocardiography evidence of low cardiac output.84 A similar incidence occurred in a high-risk population of infants <2 kg at the time of percutaneous PDA closure, where despite a targeted milrinone prophylaxis approach, 43% of the infants developed respiratory instability in the first 24 hours.68 Postoperative LV diastolic dysfunction, manifested by prolonged isovolumic relaxation time (IVRT) on early echocardiography, was associated with subsequent respiratory instability.84 This may be related to the inability of the premature LV, with an a priori diastolic dysfunction, to adapt to the acute post-closure increase in LV afterload, leading to further augmentation in LV filling pressures, secondary to pulmonary venous hypertension and oxygenation failure.84 In a more contemporary study infants who developed respiratory instability had higher end-systolic elastance both prior to and after percutaneous PDA closure. Since ventricular stiffening is associated with higher changes in pressure even in small loading perturbations, it is possible that intrinsic myocardium properties related to diastolic function may provide a basis for an increased vulnerability to changes in loading conditions.68 The subpopulation of infants with respiratory instability and LV diastolic dysfunction after percutaneous PDA closure are more likely to be hypertensive. Therefore, infants with LV diastolic dysfunction may benefit from a higher dose of milrinone to exert its lusitropic effects, although an adequately powered prospective study is needed to address this hypothesis.89 The HPA axis is crucial in regulating organ maturational events, especially the increased concentration of β-adrenergic receptors in tissues, and effective cardiovascular responsiveness to endogenous and pharmacologic vasoactive agents.90 Preterm infants are prone to life-threatening hypotension secondary to HPA axis immaturity, resulting from adrenocortical insufficiency.91 Under stress, the premature adrenal gland may not respond appropriately to elevated ACTH, producing only a blunted cortisol response.91–93 Adrenal hypoperfusion secondary to chronic ductal “steal” has been postulated to be another contributory mechanism in infants with persistent PDA.56 The combination of developmental immaturity of the HPA axis with chronic adrenal hypoperfusion is a potential contributor to early cardiovascular instability and catecholamine-resistant hypotension.56,94,95 Widespread use of stress dose hydrocortisone has not been associated with improved outcomes,96 while postoperative cortisol measurements may delay initiation of therapy. Pre-closure assessment of adrenal performance with an adrenocorticotropic hormone (ACTH) stimulation test, however, can guide early administration to appropriately selected patients.62 Therefore stress dose hydrocortisone may be indicated for patients who were receiving chronic steroid therapy preoperatively, as well as those who become symptomatic, particularly if accompanied by a failed pre-ligation ACTH stimulation test.97 The use of steroids for definitive closure has not been studied today and merits prospective evaluation. Table 18.1 describes the most common complications of surgical PDA closure.
Chapter 18: Interventional management of the patent ductus arteriosus
Introduction
Definitive PDA closure: Timing, patient selection, and staging
Impact of definitive PDA closure
Surgical PDA ligation and outcomes
Percutaneous PDA closure and outcomes
Improving risk/benefit profiles
Novel percutaneous PDA closure devices
Treatments for definitive ductal closure
Fundamental need for contemporary randomized controlled trial
PDA closure periprocedural management
Care of the preterm infant after definitive PDA closure
Physiologic changes after closure: Decrease in left ventricular preload, increase in afterload, and decrease in left ventricular output
Myocardial dysfunction
Hemodynamic changes in mesenteric and cerebral circulations
Post-closure cardiorespiratory instability and milrinone prophylaxis
Isolated post-closure respiratory instability
Hypothalamic-pituitary-adrenal (HPA) gland axis and post-closure cardiovascular instability
Surgical and percutaneous closure complications
Surgical complications
Interventional management of the patent ductus arterios us



