5. Initial Nursery Care *
Sandra L. Gardner and Jacinto A. Hernandez
Aneonate must demonstrate a condition of well-being before being considered a normal, low-risk infant. Neonatal intensive care professionals must understand the normal neonate to care for the sick neonate. This chapter discusses the initial assessment, transitional period, and gestational age characteristics that are of fundamental clinical importance for the provision of quality initial nursery care by all health care providers.
Physical, biologic, and physiologic changes occur so rapidly after birth that the assessment of the newly born can be divided into four distinctive periods: at delivery, during transition, during the first 24 hours of life, and at discharge. Each of these assessments has a specific purpose. One should consider these evaluations in relation to the age of the newborn infant (minutes, hours, days, and weeks) rather than to the location of the mother and infant in the hospital or to arbitrary nursery routines.
The evaluation at delivery is aimed at determining the condition of the infant at the time of birth (Apgar score) 6 and at detecting life-threatening emergencies. The examination during the next few hours (transition period) is used to evaluate the infant’s adjustment to extrauterine life. The complete newborn examination by a qualified health care provider should be performed at about 12 to 24 hours. 3 It is the most important examination, because many findings can be treated or complications can be avoided. Finally, the assessment/evaluation at discharge is of the utmost importance. Although it is not as detailed as the complete examination, it is aimed at establishing the infant’s readiness to leave the hospital and to be cared for by the mother. During this examination, the health care professional demonstrates the baby’s unique abilities and answers the parents’ questions. This is a good time to provide support and encouragement as the parents begin to incorporate the new member into their family.
ASSESSMENT AND CARE AT DELIVERY
Before the delivery, one should obtain pertinent facts about the pregnancy, such as parity, gravidity, fetal losses, estimated birth weight and gestational age of the fetus, and, of course, any problems present in the current pregnancy. 61 Health care providers should note whether the mother was screened for group B streptococcus and whether she received any antibiotic treatment. 3,17
During labor, one can observe the frequency and duration of contractions and the mother’s reaction to contractions. Passage of meconium, rupture of membranes, fetal distress, and other signs will alert the attendants to impending problems.
The initial respiratory effort and heart rate, part of the Apgar evaluation (see Chapter 4, Figure 4-7), are noted even before 60 seconds, because one does not wait for the 1-minute Apgar to begin resuscitative procedures if the infant is limp and not breathing (see Chapter 4). If the baby is vigorous, the care provider may place him or her on the mother’s abdomen or in her arms; Apgar assessment can be done there or in a bassinet or warmer. Scoring is repeated at 5 minutes. Under some conditions, such as prolonged resuscitation, it is helpful to have a score at 10 or 15 minutes. Between the 1- and 5-minute Apgar assessments, one systematically evaluates the baby for potential or apparent medical emergencies.
The Apgar score standardizes initial newborn assessment4 and continues to be a predictor of neonatal survival. A retrospective analysis found that for both preterm and term infants, neonatal survival increases with increasing Apgar scores; low 5-minute scores (e.g., 0 to 3) are associated with the highest risk for neonatal death. 16
With practice and experience, the professional will be able to identify approximate gestational age from the physical appearance. One quick and effective way to estimate gestational age is by measuring foot length. Foot length of appropriate-for-gestational-age preterm infants has been correlated with gestational age (Table 5-1). In short gestation (i.e., 25th to 34th week), there is a consistent, incremental increase in the mean foot length of 0.5 cm every 2 weeks. Measurement of foot length from the posterior prominence of the heel to the tip of the first (great) toe with a millimeter ruler is a rapid and simple method of assessing maturation of all newborns, even the very ill, very-low-birth-weight (VLBW) infant. With this method, as with other physical measurements of gestational age, one must consider the standard deviation in interpreting the results.
| SD, Standard deviation. | |||||
| *Applies to both male and female infants. | |||||
| From Hernandez JA, Lazarte R, Pisano D, et al: Foot length and gestational age in the very-low-birth-weight infant, The Children’s Hospital Pediatric Update, September 1987, p 4. | |||||
| Gestational Age (wk) | Foot Length (cm) | ||||
|---|---|---|---|---|---|
| No. of Infants | Mean | Median | SD | Range | |
| 24 | 6 | 4.22 | 4.1 | 0.17 | 3.8-4.4 |
| 25 | 12 | 4.5 | 4.5 | 0.08 | 4.4-4.6 |
| 26 | 16 | 4.72 | 4.7 | 0.07 | 4.65-4.9 |
| 27 | 19 | 4.99 | 5.0 | 0.14 | 4.8-5.2 |
| 28 | 18 | 5.23 | 5.2 | 0.13 | 5.0-5.5 |
| 29 | 22 | 5.47 | 5.4 | 0.129 | 5.3-5.7 |
| 30 | 27 | 5.75 | 5.75 | 0.23 | 5.6-6.2 |
| 31 | 24 | 5.95 | 6.0 | 0.19 | 5.7-6.23 |
| 32 | 21 | 6.22 | 6.2 | 0.13 | 6.0-6.4 |
| 33 | 25 | 6.5 | 6.5 | 0.26 | 6.3-6.9 |
| 34 | 24 | 6.77 | 6.8 | 0.20 | 6.5-7.1 |
| 35 | 20 | 7.1 | 7.0 | 0.15 | 6.8-7.3 |
| 36 | 22 | 7.27 | 7.27 | 0.21 | 7.0-7.6 |
| 37 | 24 | 7.51 | 7.5 | 0.24 | 7.4-8.0 |
| 38 | 40 | 7.92 | 8.0 | 0.23 | 7.6-8.3 |
| 39 | 42 | 8.22 | 8.3 | 0.32 | 7.9-8.6 |
| 40 | 56 | 8.6 | 8.7 | 0.37 | 8.2-8.9 |
| 41 | 22 | 8.75 | 8.9 | 0.30 | 8.3-9.1 |
| 42 | 12 | 9.1 | 9.2 | 0.33 | 8.7-9.3 |
| 43 | 8 | 9.27 | 9.3 | 0.25 | 8.9-9.6 |
After turning the infant to a prone position, further inspection will reveal congenital abnormalities such as spina bifida, imperforate anus, skeletal abnormalities, or genital defects. Internal abnormalities should be suspected if an “empty” or scaphoid abdomen (diaphragmatic hernia) or profuse oral or nasopharyngeal mucus (tracheoesophageal [TE] fistula) is present. Choanal atresia may present as apnea after respirations have been established. If closing the infant’s mouth results in cyanosis and/or apnea, this is a positive test result for choanal atresia. Examination of the umbilical cord and vessels may give a clue to other abnormalities. The size and amount of Wharton’s jelly, especially if the cord is thin, suggest problems in intrauterine nutrition. A single umbilical artery may be a clue to other anomalies (e.g., genitourinary, gastrointestinal, cardiovascular, central nervous system [CNS], twin-to-twin transfusion, and respiratory).
EVALUATION AND CARE DURING THE TRANSITIONAL PERIOD
Physiologic Changes and Clinical Stages
The initial evaluation, assessment, and, management of a newborn must be directed toward promoting and facilitating normal adaptation to extrauterine life and early detection of significant health problems so that they can be evaluated and treated promptly and appropriately. 47
The obligatory change of environment at birth necessitates adjustment to extrauterine environment so that the newborn experiences a complex series of biologic, physiologic, and metabolic changes. These changes are essential for survival. Every infant must complete this process of transition successfully to survive in the extrauterine environment. For a small percentage of newborns, transition is never achieved; for a slightly larger number, transition is delayed or complicated. For most newborns, transition is so smooth it appears uneventful.
With the first breath of life and the cutting of the umbilical cord, all neonates begin the transition from intrauterine to extrauterine life. Three major changes take place at birth. First, fluid in the alveoli is reabsorbed, allowing diffusion of air into the surrounding blood vessels. Second, because the umbilical arteries and vein are clamped, the low-resistance placental circuit is gone and systemic blood pressure increases. Third, pulmonary vascular resistance is decreased as a result of mechanical distention of the alveoli and increased oxygen content in the alveoli. Oxygen is a potent pulmonary vasodilator.
During the first few hours after birth, the normal newborn progresses through a fairly predictable sequence of events, recovering from the stress of birth and adapting to extrauterine life. Intrapartum and immediate neonatal events result in sympathetic discharges reflected in changes in heart rate, color, respiration, motor activity, gastrointestinal function, and temperature. 47Awake and sleep states affect a neonate’s behavior and ability to respond to the environment. A newborn may go from one state to another quite frequently in the nursery and at home (see Critical Findings: Newborn States and Considerations for Caregiving in Chapter 13). Figure 5-1 shows the classic description by Desmond of the transitional period, which includes the three stages shown in Box 5-1. Failure to establish this pattern of transition requires careful observation and investigation.
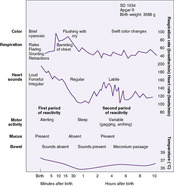 |
| FIGURE 5-1 (From Desmond MM, Rudolph AJ, Phitaksphraiwan P, et al: The transitional care nursery: a mechanism for preventive medicine in the newborn, Pediatr Clin North Am 13:651, 1966.) |
BOX 5-1




First Stage (0-30 min) = First Period of Reactivity
• Rapid increase in heart rate to the range of 160-180 beats/min (0-15 min)
• Gradual decrease in heart rate over 30 min to baseline rate between 100-120 beats/min
• Irregular respirations (first 15 min), peak respiratory rates between 60-80 breaths/min
• Rales present on auscultation
• Grunting, flaring, and retractions may be noted, and brief periods of apnea (<10 sec in duration)
• Plethora
• Alert with spontaneous startle reactions, gustatory movements, tremors, crying, and side-to-side head movements
• Decrease in body temperature
• Generalized increase in motor activity, with increased muscle tone
• Bowel sounds absent, and abdomen distended
• Production of saliva minimal
Second Stage (30 min–2 hr) = Period of Decreased Responsiveness
• Newborn either sleeps or has a marked decrease in motor activity
• Muscle tone returns to normal, but responsiveness is diminished
• Fast, shallow, synchronous breathing (60 breaths/min) without dyspnea occurs
• Newborn’s color is pale but pink with excellent perfusion and capillary refill
• Increase in anterior-posterior diameter (barreling) of the chest is usually present
• Heart rate decreases into the range of 100-120 beats/min or lower; the newborn is relatively less responsive to external stimuli
• Abdomen is rounded, and bowel sounds are audible; peristaltic waves may be visible, and meconium may be passed
• Oral mucus is absent
• Spontaneous jerks and twitches are common, but the newborn quickly returns to rest
Third Stage (2-8 hr) = Second Period of Reactivity
• Return of and possible exaggeration of responsiveness
• Labile heart rate: periods of tachycardia
• Brief periods of rapid respirations
• Abrupt changes in tone, color, and bowel sounds
• Possible prominence of oral mucus; gagging and vomiting not unusual
• Possible clearing of meconium from the bowel
• Increased responsiveness to endogenous and exogenous stimuli
• Newborn hunger cues; quiet alert periods when maternal bonding is established
Modified from Hernandez JA, Thilo E: Routine care of the full-term newborn. In Osborn LC, DeWitt TG, First LR, et al, editors: Pediatrics, St Louis, 2005, Mosby.
Management of the Newborn During Transition
Traditionally, care in “normal newborn” nurseries was based on the optimistic assumption that most newborns have no difficulty with transition after birth and that term infants, in particular, do exceedingly well. With this philosophy, nursery care was geared toward the 85% to 90% of newborns who do well rather than the 10% to 15% with transitional complications. 47 Modern nursery care recognizes the complexity of transitioning to extrauterine life and the reality of serious disease even in term newborns.
NEWBORN SHOULD BE TREATED AS A RECOVERY PATIENT
During the immediate neonatal period, an “intensive care” concept has been introduced into care of the newly born. All newborns are to be cared for, regarded, and observed as recovering patients until they have successfully completed a smooth transition.
SKILLED PROVIDERS SHOULD CARE FOR THE NEWBORN
Current standards of care3require skilled health care providers (24 hours/day) to care for newborns during the first minutes after birth (e.g., in the delivery room; birth center) and in the follow-up period (e.g., in the birth room; mother-baby area; newborn nursery). 47 All personnel caring for the newborn must be familiar with the transitional changes after birth and deviations from normal transitional events. After a normal, low-risk pregnancy and birth, primary evaluation and care of the newborn must be provided by educated, professional neonatal nurses who consult advanced practice nurses and/or physician(s) when appropriate. 3,47
STANDARDS FOR ROUTINE CARE AND PHYSIOLOGIC MONITORING DURING TRANSITION MUST BE MAINTAINED
Both parent-infant bonding and careful neonatal monitoring during the transition period should be addressed in delivery/birth room and nursery routines. After birth, the stable, pink newborn whose Apgar score is greater than 7 at 5 minutes can be rewrapped in warm, dry blankets and given to the parents to hold. Early breast feeding and skin-to-skin contact are acceptable if the neonate is stable and continuous observation is provided. After birth, at 15-minute intervals, every newborn must be assessed for general condition, respiratory effort, color, muscle tone, and temperature; all assessments must be documented.47
When the neonate is admitted to the transition nursery (or recovered with the parents), anthropometric measurements (e.g., weight, length, head circumference) and vital signs are evaluated and recorded. Routine care in Table 5-2 includes glucose screening, eye prophylaxis, and administration of vitamin K 1. By 30 minutes of age, every newborn, regardless of where the baby is being cared for, must be examined by a neonatal nurse. During the first 6 hours after birth, heart rate, respirations, blood pressure, degree of alertness, and color of skin and mucous membranes should be assessed frequently and the findings recorded. This period is when clinical signs of the most threatening infections, cardiopulmonary diseases, and major congenital abnormalities appear. Table 5-3 presents a useful scoring system for assessing the pattern of respirations for signs of respiratory distress; findings should be documented. For indirectly measured blood pressure, the range of normal is 65 to 95 mm Hg systolic and 30 to 60 mm Hg diastolic, with an average mean blood pressure of 50 to 55 mm Hg in term infants. The blood pressure value will steadily increase from birth over the transitional period. 47
| IM, Intramuscular; PO, orally; POC, point-of-care. | |||
| *Routine care is required wherever (e.g., labor-delivery-recovery; labor-delivery-recovery-postpartum; birth center; mother-baby unit; nursery) the newly born infant is cared for after birth. | |||
| Modified from Hernandez JA, Thilo E: Routine care of the full-term newborn. In Osborn LC, DeWitt TG, First LR, et al, editors: Pediatrics, St Louis, 2005, Mosby. | |||
| Routine Care | Time | Drug/Dose | Comments |
|---|---|---|---|
| Glucose screening (see 15) | At 30-60 minutes of age | By POC glucometer device Abnormal screen: glucose <40 mg/dL | |
| Eye prophylaxis | Within 1 hour of age | Erythromycin (0.5%) or tetracycline (1%) eye ointment: apply ribbon in each conjunctival sac | Eye prophylaxis for ophthalmia neonatorum Bacteriocidal effect depends on tissue concentration of drug and microorganisms |
| Vitamin K 1 | Within 1 hour of age | 0.5-1 mg IM as a single dose for infants <1.5 kg or >1.5 kg or | Prophylaxis for hemorrhagic disease of the newborn. Vitamin K concentrations are physiologically low in breast milk so that exclusively breast-fed infants are at increased risk for vitamin K deficiency as are infants with fat malabsorption (e.g., biliary atresia, cystic fibrosis, alpha 1-antitrypsin deficiency) and prolonged treatment with antibiotics. (See Chapter 12 for use of sucrose and topical analgesia to be used for pain relief for injections.) |
| 2 mg PO | Repeated oral dosing (e.g., first feed, 1 week, 4 weeks, 8 weeks) is necessary; increased risk for late-onset hemorrhagic disease when infant receives only one dose. PO intake contraindicated in preterms, sick infants with diarrhea or cholestasis or receiving antibiotics | ||
| F io2, Fraction of inspired oxygen; RDS, respiratory distress syndrome. | |||
| *The respiratory distress syndrome score is the sum of the individual scores for each of the five observations. | |||
| †Air entry represents the quality of inspiratory breath sounds as heard in the midaxillary line. | |||
| From Downes JJ, Vidyasager DD, Boggs TR, et al: Respiratory distress syndrome in newborn infants: I. New clinical scoring system (RDS score) with acid-base and blood-gas correlates, Clin Pediatr 9:325, 1970. | |||
| 0 | 1 | 2 | |
|---|---|---|---|
| Respiratory rate (breaths/min) | 60 | 60-80 | >80 or apneic episode |
| Cyanosis | None | In room air | In 40% F io2 |
| Retractions | None | Mild | Moderate to severe |
| Grunting | None | Audible with stethoscope | Audible without stethoscope |
| Air entry† | Clear | Delayed or decreased | Barely audible |
Abnormal Transition
Regardless of gestational age or route of delivery, the sequence of clinical behavior just described is common to all well newborns. Preterm infants may exhibit variations in the duration of the transitional phases—shorter phase 1 or longer phase 2—but the patterns are similar. Knowledge of the normal changes occurring during transition enables early recognition of a newborn who is not making a normal extrauterine adaptation.47
Failure to make a normal transition to extrauterine life may result from obstetric anesthesia or analgesia, neonatal illness, or stress such as perinatal asphyxia and its sequelae. If the infant’s pulse, respirations, color, and activity have not stabilized within the normal ranges after 1 hour of life, a problem should be suspected and investigated.
Observation for risk factors for abnormal transition is essential. A variety of conditions may result in significant deviation from the normal sequence of events during transition. Table 5-4 lists factors that may alter the sequence or pattern of changes expected to occur after birth and that result in either a healthy newborn or a newborn with significant illness. When caring for a newborn with an altered or delayed transition, the factors in Table 5-4 must be considered. The health care provider’s challenge is to discriminate between signs of diseases that produce an ill newborn from the dynamic, rapidly changing features that accompany the physiologic adjustments of normal or altered transition but that still result in a healthy neonate.47Box 5-2 lists clinical manifestations of abnormal transition.
| Maternal factors | Chronic hypertension Pregnancy-induced hypertension Diabetes mellitus Renal disease Infection Abuse of tobacco, alcohol, or illicit drugs Collagen vascular diseases Hemizygous hemoglobinopathies Certain maternal medications |
| Obstetric factors | Rh or other isoimmunization Fetal growth restriction Decreased fetal movements Multiple gestation Oligohydramnios or polyhydramnios Premature rupture of membranes Third-trimester bleeding Delivery by cesarean section |
| Neonatal factors | Prematurity (<37 weeks) Postmaturity (>42 weeks) Small for gestational age Large for gestational age Infection Metabolic abnormalities Birth trauma Major malformations Anemia Apgar 0-4 at 1 minute or need for resuscitation at delivery |
BOX 5-2
• Persistent tachypnea, flaring, grunting, and retractions (respiratory score >4; duration >first hour of life); fixed bradycardia
• Diffuse and persistent rales, retractions, flaring, and grunting (respiratory score >4; duration >first hour of life)
• Persistent cyanosis (persistent oxygen saturation <90% in room air) and prolonged requirements for supplemental oxygen (after 2-3 hr of age)
• Episodes of prolonged apnea (>20 sec) and bradycardia (<80 beats/min)
• Marked pallor or ruddiness
• Temperature instability, persistently (after 2-3 hr of age) low temperature (<36.5 ° C)
• Poor capillary filling (>3 sec) and blood pressure instability
• Unusual neurologic behavior (lethargy, decreased activity with marked and persistent hypotonia, irritability, excessive tremors and jitteriness)
• Excessive oral secretions, drooling, and choking/coughing spells, cyanosis
Modified from Hernandez JA, Thilo E: Routine care of the full-term newborn. In Osborn LC, DeWitt TG, First LR, et al, editors: Pediatrics, St Louis, 2005, Mosby.
PHYSICAL ASSESSMENT OF THE NEWBORN
Data Collection
HISTORY
Good perinatal care requires the identification of social, demographic, and medical-obstetric risk factors that correlate with fetal outcome. This must be an ongoing process, because high-risk patients may be identified on the first prenatal visit, during follow-up prenatal visits, or not until the intrapartum and postpartum periods. Review of the perinatal history is important in determining significant factors for neonatal health management. Identification of an at-risk maternal situation is essential to plan and organize care for an at-risk neonate. Review of the perinatal history includes antepartum and intrapartum events (see Chapter 2) and events of the neonatal course, such as normal or abnormal transition, timing and onset of symptoms, and the ability to feed.
SIGNS AND SYMPTOMS
Unlike the verbalizing adult patient, the nonverbal neonate communicates needs primarily by behavior. Through objective observations and evaluations, the neonatal care provider interprets this behavior into information about the individual infant’s condition. Assessment of the neonate includes the following:
• Estimation of gestational age
• Physical examination
• Neurologic examination
• Brazelton examination
All care providers must not only be familiar with these tools but also be proficient in performing and interpreting them.
Assessment of Growth and Gestational Age
An assessment of gestational age should be done on all newborns to assign a newborn classification, determine neonatal mortality risk, generate a problem list of potential morbidities, and quickly initiate appropriate screening procedures and/or interventions for recognized morbidities.61 Gestational age can be assessed by obstetric methods and by pediatric methods.
The obstetric methods for determining maturity will have already been performed by the time the newborn reaches the nursery. However, the newborn’s care providers should be familiar with dating a pregnancy. Dating the last menstrual period (LMP) could be the most accurate method if the mother is sure of the dates of her last menstrual period. Some women will have spotting or even a light period after becoming pregnant, making them unsure of the time of conception. The use of birth control pills may also make the time of ovulation and conception unknown; therefore pregnancy tests are useful in confirming the pregnancy but not the timing of conception.
The most accurate assessment is the ultrasonographic examination during the first trimester. Ultrasonography is preferred because it confirms conception, assesses gestation, and evaluates fetal growth.
Pediatric methods of determining gestational age are based on physical characteristics and neurologic examination. Within 2 hours of birth, 3every newborn should have an assessment of gestational age by physical characteristics. Numerous tables, charts, and graphs are available for determining gestation. Some tables are more subjective than others, but at least one form should be used by all nurseries.
Three of the available charts for determining gestational age by physical characteristics are shown inFIGURE 5-2, FIGURE 5-3 and FIGURE 5-4Figure 5-2 does not place much emphasis on the neurologic assessment, which may not be valid in the first 24 hours because of birth recovery. 2 To use this chart, an X or ditto marks (″) are placed in each appropriate slot. Then an age is assigned according to a line drawn through the point where most of the marks have been placed. The disadvantage of this system is the subjectivity of the chart; the advantage is that items relate to gestational age, not a score. The examiner must therefore be experienced to offset the possibility of error in the chart.
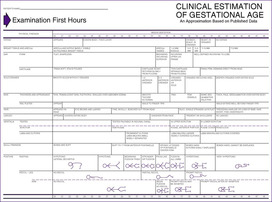 |
| FIGURE 5-2 (From Kempe CH, Silver HK, O’Brien D: Current pediatric diagnosis and treatment, ed 3, Los Altos, Calif, 1974, Lange Medical.) |
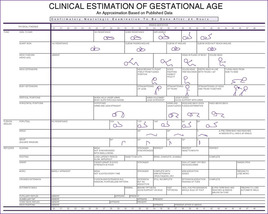 |
| FIGURE 5-3 (From Kempe CH, Silver HK, O’Brien D: Current pediatric diagnosis and treatment, ed 3, Los Altos, Calif, 1974, Lange Medical.) |
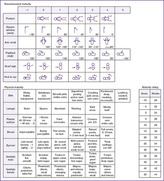 |
| FIGURE 5-4 (From Ballard JL, Khoury JC, Wedig K, et al: New Ballard score, expanded to include extremely premature infants, J Pediatr 119:417, 1991.) |
Figure 5-4 incorporates physical maturity and neuromuscular maturity on an equal basis. An X is placed in the appropriate box for each category. The score for the neuromuscular and physical maturity is added and noted under the maturity rating column. Weeks of gestation are assigned according to the maturity rating score.
Accuracy in estimation of gestational age is important, because for VLBW infants, small differences in gestational age result in large differences in outcome and may be a criterion in decision making by parents and professionals as they decide whether comfort care or intensive care is used. 25,58 Research has shown that estimation of gestational age in very immature preterm infants is inaccurate. 25 For preterm infants of 22 to 28 weeks’ gestation, estimates of gestational age (by the scoring system shown in Figure 5-4) exceeded the gestational age (by dates) by 1.3 to 3.3 weeks. 25 These inaccuracies must be considered in decision making, and better scoring systems are needed. 25,58
To use these charts accurately, the examiner must assess the following physical characteristics.61,84
Vernix
At 20 to 24 weeks, vernix is produced by sebaceous glands. Note the amount and distribution of vernix on the baby’s skin (best done in the delivery room). Vernix is high in fat content and protects the skin from the aqueous amniotic fluid and bacteria. At 36 weeks, the white, cheeselike material begins to decrease and disappears by 41 weeks.
Skin
In early gestation, the skin of the fetus is very transparent and veins are easily seen. As gestation progresses, the skin becomes tougher, thicker, and less transparent.
By 37 weeks, very few vessels are visible. From 36 weeks to delivery, fat deposits begin to form and grow. In a postterm infant, desquamation will be prominent at the ankles, wrists, and possibly palms and soles. As gestation progresses, the loss of vernix and subcutaneous tissue causes wrinkling. Note skin turgor, color, texture, and the prominence of vessels, especially on the abdomen.
Lanugo
At 20 weeks, fine, downy hair (lanugo) appears over the entire body of the fetus. At 28 weeks, it begins to disappear around the face and anterior trunk. At term, a few patches of lanugo may still be present over the shoulders. Note the distribution of lanugo, first on the face and anterior trunk and then on the rest of the body.
Hair on the Head
Hair appears on the head at 20 weeks. At 20 to 23 weeks, the eyelashes and eyebrows develop. From 28 to 36 weeks, the hair is fine and woolly and sticks together. It appears disheveled and sticks out in bunches from the head. At term, the hair lies flat on the head, it feels silky, and single strands are identifiable. Note the quality and distribution of the hair, and feel its texture. Scalp hair abnormalities (e.g., growth pattern, hypopigmentation, quantity, distribution, texture) may be external markers of genetic, metabolic, and neurologic disorders.
Sole Creases
Sole creases develop from toe to heel, progressing with gestational age. An infant with intrauterine growth restriction (IUGR) and early loss of vernix may have more sole creases than expected. By 12 hours after birth, the skin has dried to a point that sole creases are no longer a valid indicator of gestational age. Note the development of sole creases as they progress from the superior to inferior aspects of the foot (Figure 5-5).
Eyes
In the third month of fetal life, the eyelids fuse; they reopen between 26 and 30 weeks. In neonates of 27 to 34 weeks’ gestation, examination of the anterior vascular capsule of the lens is useful in assessing gestational age. Gestational age is determined by assessing the level of remaining embryonic vessels on the lens (Figure 5-6). Before 27 weeks, the hazy cornea prevents visualization of the vascular system. After 34 weeks, only remnants of the vascular system are visible. Because rapid atrophy occurs in the vascular system, an ophthalmoscopic examination should be performed during the first physical examination or within 24 to 48 hours after birth.
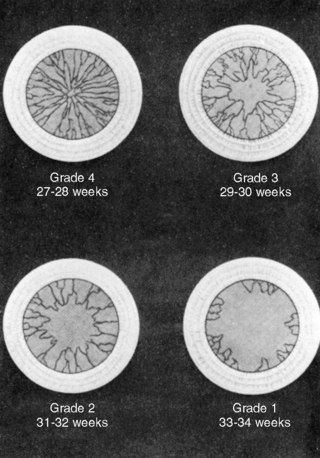 |
| FIGURE 5-6 (From Hittner H, Hirsch NJ, Rudolph AJ: Assessment of gestational age by examination of the anterior vascular capsule of the lens, J Pediatr 91:455, 1977.) |
Ears
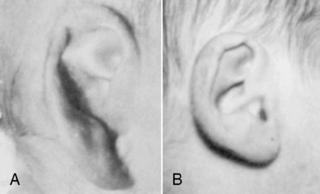
Before 34 weeks, the pinna of the ear is a slightly formed, cartilage-free double thickness of skin. When it is folded, it remains folded. As gestation progresses, the pinna develops more cartilage, resulting in better form, so that it recoils when folded (Figure 5-7). Check ear recoil by folding the ear in half or into a three-corner-hat shape. Consistently folding it the same way helps the care provider develop a baseline for judging maturity. Note the form and cartilage development of the ear. Examine both ears to be sure they are the same and without defects.
Breast Development
Breast development is the result of the growth of glandular tissue related to high maternal estrogen levels and fat deposition. The areola is raised in an infant of 34 weeks’ gestation. Note the size, shape, and placement of both breasts. Palpate the breast nodule and determine its size. If the infant is growth restricted, breast size may be less than expected at term.
Genitalia
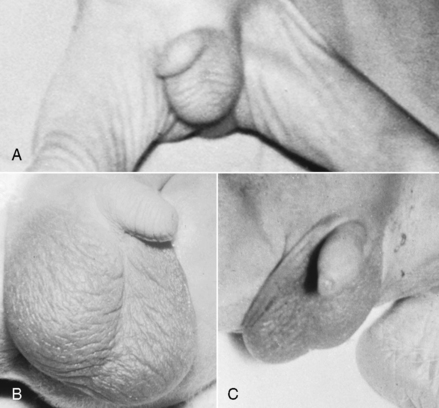
Male Genitalia
At 28 weeks, the testes begin to descend from the abdomen. By 37 weeks, they are high in the scrotum. By 40 weeks, the testes are completely descended and the scrotum is covered with rugae. As gestation progresses, the scrotum becomes more pendulous (Figure 5-8). Note the presence of rugae on the scrotum and its size in relation to the position of the testes. When examining the baby for descended testes, put the fingers of one hand over the inguinal canal to prevent the testes from ascending into the abdominal cavity and palpate the scrotal sac with the other hand.
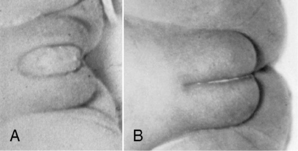
Female Genitalia
Early in the female’s gestation, the clitoris is prominent with small and widely separated labia. By 40 weeks, the fat deposits have increased in size so that the labia majora completely cover the labia minora (Figure 5-9). Note the labial development in relation to the prominence of the clitoris.
Newborn Classifications
The clinical estimate of gestation is defined by weeks of gestation into the following categories (Figures 5-10and5-11):
 |
| FIGURE 5-10 (From Johnson JL, Merenstein G, Coll J, et al: Colorado intrauterine growth curve 1980-1992: the new Lubchenco growth curve, Pediatr Res 35:274A, 1994.) |
 |
| FIGURE 5-11 (From Lubchenco LO: The high-risk infant, Philadelphia, 1976, Saunders.) |
• Full-term (F)—38 through 41 completed weeks
• Postterm (PO)—42 weeks or more
Intrauterine growth curves for the 10th and 90th percentiles are represented in Figure 5-10. Small-for- gestational-age (SGA) infants are those below the 10th percentile. Appropriate-for-gestational-age (AGA) infants are those between the 10th and 90th percentiles. Large-for-gestational-age (LGA) infants are above the 90th percentile. Based on birth weight, the infant’s intrauterine growth will be SGA, AGA, or LGA.
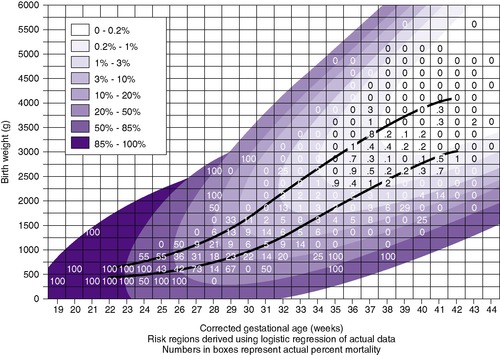
Using the clinical estimate of gestational age (in weeks) and the birth weight (in grams), one determines the newborn’s classification. The combined gestational age and weight criteria shown inFigure 5-10form nine possible newborn classifications: preterm, full-term, and postterm large-for-gestational-age (PRLGA, FLGA, and POLGA); preterm, full-term, and postterm appropriate-for-gestational-age (PRAGA, FAGA, and POAGA); and preterm, full-term, and postterm small-for-gestational-age (PRSGA, FSGA, and POSGA).
UsingFigure 5-10, it is possible to plot the newborn weight in grams against the clinical gestational age (marking an X on the chart) by determining to which of the nine categories the newborn belongs and then classifying and noting his or her classification on the record.
Neonatal Mortality Risk
Neonatal mortality risk (NMR), the chance of dying in the neonatal period, can be determined from graphs such as that shown inFigure 5-10and is based on birth weight and gestational age. This figure is based on the Lubchenco Perinatal Database, University of Colorado Hospital, 1980 to 1992. On the chart, the area of least risk is the FAGA infant. Deviations from this area of least risk in relation to either weight or gestational age increase the newborn’s mortality risk.
Mortality has changed over time because an increasingly physiologic basis of care has been used, coupled with sophisticated professional care, technology, transport systems, and aggressive management to handle increasingly at-risk populations. For example, before recent years, LGA infants were at increased risk for mortality; this is no longer true because of earlier recognition and better obstetric management (see Chapter 2). Babies with greater than 10% risk for neonatal mortality usually require level II or III care. Note the infant’s NMR on the chart (see Figure 5-10), and insert an entry in the newborn record. N ote: To determine the appropriate NMR, read to the right of the vertical lines and above the horizontal line.
Examination of NMR in Figure 5-10 also reveals that two infants with the same birth weight but with different gestational ages may have very different risks for death. For example, infant A may have a birth weight of 2000 g and a gestational age of 33 weeks. Plotting these values on Figure 5-10, one determines the NMR for this infant at 2%. Infant B, on the other hand, may also weigh 2000 g but have a gestational age of 39 weeks. The infant’s risk is 0%. Infant A thus has a mortality risk 10 times greater than that of infant B, even though they have the same birth weight.
Within the National Institutes of Child Health and Human Development (NICHD) Neonatal Research Network, mortality for newborns weighing 501 to 1500 grams decreased from 23% (1987-88), to 17% (1993-94), to 14% (1999-2000). However, within each birth weight category, survival free of major morbidity (e.g., chronic lung disease/bronchopulmonary dysplasia [CLD/BPD], necrotizing enterocolitis [NEC], grade 3 or 4 intraventricular hemorrhage [IVH]) did not change significantly. Because mortality (and morbidity) are highest in infants of the lowest birth weights and gestational ages, VLBW and extremely low birth weight (ELBW) infants have better outcomes when born in a facility that can provide the appropriate subspecialty care.11,18,57,88 Infants requiring transfer to another facility have a greater risk for morbidity and mortality when compared with infants born in a tertiary care center; this advantage is inversely related to gestational age. 57These research findings have prompted recommendations that high-risk infants (e.g., <32 weeks’ gestational age) be born in a facility capable of providing the anticipated appropriate level of neonatal intensive care.3
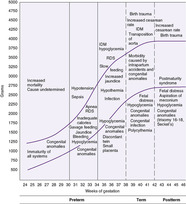
Neonatal Morbidity Risk
Neonatal morbidity risk (see Figure 5-11) is determined by deviations of intrauterine growth and newborn classification. Classification of the newborn assists in identification, observation, screening, and treatment of the most commonly occurring problems. For every newborn, formulate a problem list based on the morbidities common to the newborn classification. Observe, screen, intervene, and refer as necessary to prevent complications.
SGA/IUGR infants are at increased risk for morbidities (e.g., perinatal depression, hypothermia, hypoglycemia, polycythemia, infection) immediately after birth. There is also an association between size at birth, altered physiologic development, and long-term developmental and health problems (especially heart disease and stroke).21
LATE-PRETERM (“NEAR-TERM”) INFANTS
In the United States, 39 weeks has become the most common length of gestation. 24 Since 1981, preterm birth rate has increased by 30%. 48 In 2004, the prematurity rate in the United States was 12.5%. Two thirds of the increase in the rate was due to increasing rates of “near-term” births, so that 8.5% of all U.S. births for 2002 were neonates of 34 to 36  weeks of gestation. 48 A 2006 March of Dimes study showed that 75% of all singleton preterm births are 34 to 36 weeks’ gestation and that 36 completed weeks of gestation accounts for 40.1% of all singleton preterm births. 24 Because these infants are at increased risk for health and developmental problems when compared with full-term infants, this trend has been called a growing public health problem. 48
weeks of gestation. 48 A 2006 March of Dimes study showed that 75% of all singleton preterm births are 34 to 36 weeks’ gestation and that 36 completed weeks of gestation accounts for 40.1% of all singleton preterm births. 24 Because these infants are at increased risk for health and developmental problems when compared with full-term infants, this trend has been called a growing public health problem. 48
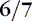 weeks of gestation. 48 A 2006 March of Dimes study showed that 75% of all singleton preterm births are 34 to 36 weeks’ gestation and that 36 completed weeks of gestation accounts for 40.1% of all singleton preterm births. 24 Because these infants are at increased risk for health and developmental problems when compared with full-term infants, this trend has been called a growing public health problem. 48
weeks of gestation. 48 A 2006 March of Dimes study showed that 75% of all singleton preterm births are 34 to 36 weeks’ gestation and that 36 completed weeks of gestation accounts for 40.1% of all singleton preterm births. 24 Because these infants are at increased risk for health and developmental problems when compared with full-term infants, this trend has been called a growing public health problem. 48Historically, infants of 34 to 38 weeks’ gestational age have been considered “slightly preterm”59 or “borderline prematures.”77 Use of this terminology reminded heath care providers to have a higher index of suspicion related to morbidities when compared with term infants. In the early 1990s, the terminology “near term” began to be used in the literature. These “near term” infants not only are cared for in special care nurseries but also are found in normal newborn nurseries, mother-baby care, and labor/delivery/recovery/postpartum (LDR/LDRP), 40 because they are “considered functionally full term and management decisions are made accordingly.”87,p.372
In 2005 at the National Institutes of Health (NIH) meeting, 74 a proposal was made to use the term “late-preterm” rather than “near-term” to reflect the increased morbidity (and mortality) of this group of biologically and physiologically immature neonates. 27 A retrospective chart review of late-preterm infants showed that they have significantly more morbidities. At 34 to 35 weeks, all the common morbidities are more frequent: (1) respiratory distress syndrome (RDS) is twice as common after cesarean section, (2) perinatal depression, (3) hypoglycemia, (4) jaundice, (5) sepsis, (6) feeding problems, and (7) apnea. 8 In this same study, RDS was five times more common at 37 to 38 weeks’ gestation after cesarean section. 8This is consistent with other studies of elective cesarean section, as follows:
• Those born at 37 to 38 weeks’ gestation were 120 times more likely to receive ventilator support for surfactant deficiency than infants born at 39 to 41 weeks. 64
• Risk for respiratory morbidity is increased at any gestation less than 40 weeks (at 39 weeks, risk is doubled but not statistically significant; at 38 weeks, risk is triple; at 37 weeks, risk is fourfold). 46
Another study of “late-preterm” infants (i.e., 35-36 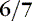 weeks’ gestation) showed that they had more clinical problems, longer lengths of stay, and higher costs when compared with full-term newborns. 87Table 5-5 shows the difference in occurrence of morbidities between late-preterm infants (with good Apgar scores and appropriate size) and their full-term contemporaries. In addition to more acute respiratory morbidity, research shows late-preterms have more BPD/CLD, neurologic complications, rehospitalizations, and mortality. 10,28,31,82,83
weeks’ gestation) showed that they had more clinical problems, longer lengths of stay, and higher costs when compared with full-term newborns. 87Table 5-5 shows the difference in occurrence of morbidities between late-preterm infants (with good Apgar scores and appropriate size) and their full-term contemporaries. In addition to more acute respiratory morbidity, research shows late-preterms have more BPD/CLD, neurologic complications, rehospitalizations, and mortality. 10,28,31,82,83
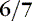 weeks’ gestation) showed that they had more clinical problems, longer lengths of stay, and higher costs when compared with full-term newborns. 87Table 5-5 shows the difference in occurrence of morbidities between late-preterm infants (with good Apgar scores and appropriate size) and their full-term contemporaries. In addition to more acute respiratory morbidity, research shows late-preterms have more BPD/CLD, neurologic complications, rehospitalizations, and mortality. 10,28,31,82,83
weeks’ gestation) showed that they had more clinical problems, longer lengths of stay, and higher costs when compared with full-term newborns. 87Table 5-5 shows the difference in occurrence of morbidities between late-preterm infants (with good Apgar scores and appropriate size) and their full-term contemporaries. In addition to more acute respiratory morbidity, research shows late-preterms have more BPD/CLD, neurologic complications, rehospitalizations, and mortality. 10,28,31,82,83| Frequency | ||
|---|---|---|
| Morbidity | Late Preterm (“Near Term”) | Full Term |
| Temperature instability | 10% | 0% |
| Hypoglycemia | 15.6% | 5.3% |
| Intravenous infusions | 26.7% | 5.3% |
| Respiratory distress | 28.9% | 4.2% |
| Jaundice | 54.4% | 37.9% |
| Apnea/bradycardia | 4.4% | 0% |
| Sepsis evaluation | 36.7% | 12.6% |
| Poor feeding | 76% | 28.6% |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




 weeks
weeks